We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1767
From Proteopedia
(Difference between revisions)
| Line 19: | Line 19: | ||
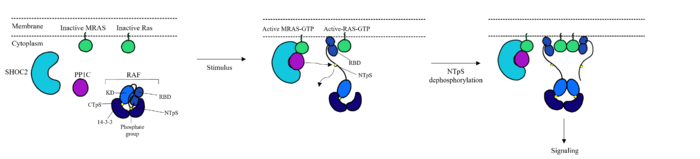
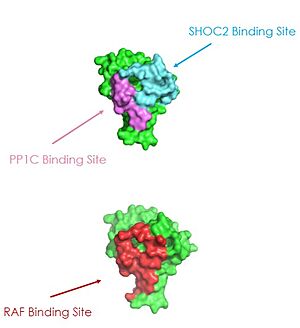
<scene name='95/952695/Shoc2intro/1'>SHOC2</scene> is essential for complex formation. It is a crescent shaped complex that serves as a bridge for PP1C and MRAS, maximizing interaction between the three subunits of the SMP complex <ref name="Hauseman">PMID:35830882</ref>. SHOC2 contains a large leucine rich region (LRR) that provides stability and localizes subunit PP1C to the membrane<ref name="Liau">PMID: 35768504</ref>. SHOC2 only undergoes a <scene name='95/952693/Shoc2_gtp_bound_vs_gdp_bound/7'>6° conformational change</scene> when PP1C and MRAS bind showing it is a scaffolding protein that provides a favorable interface for complex formation<ref name="Liau">PMID: 35768504</ref>. SHOC2 depletion is being studied as a therapeutic approach for RAS-driven cancers due to large scale interactions of the subunits being made possible by SHOC2 <ref name="Kwon">PMID: 35831509</ref>. As shown in '''Figure 1 '''SHOC2 and PP1C first engage in binding with each other via an N-terminal <scene name='95/952695/Rvxf_motif/2'>RVXF Motif</scene> on SHOC2 that is complimentary to a binding sequence on PP1C. SHOC2 residues <scene name='95/952695/Shoc2_highlighted_residues/1'>V64 and F66</scene> embed in the complimentary region of PP1C, enhancing SHOC2 affinity for PP1C. SHOC2 binds MRAS-GTP through β strands of a LRR that interacts with a [https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Physical_Properties_of_Matter/Atomic_and_Molecular_Properties/Intermolecular_Forces/Hydrophobic_Interactions. hydrophonic] region of MRAS-GTP further stabilizing the complex<ref name="Kwon">PMID: 35831509</ref>. | <scene name='95/952695/Shoc2intro/1'>SHOC2</scene> is essential for complex formation. It is a crescent shaped complex that serves as a bridge for PP1C and MRAS, maximizing interaction between the three subunits of the SMP complex <ref name="Hauseman">PMID:35830882</ref>. SHOC2 contains a large leucine rich region (LRR) that provides stability and localizes subunit PP1C to the membrane<ref name="Liau">PMID: 35768504</ref>. SHOC2 only undergoes a <scene name='95/952693/Shoc2_gtp_bound_vs_gdp_bound/7'>6° conformational change</scene> when PP1C and MRAS bind showing it is a scaffolding protein that provides a favorable interface for complex formation<ref name="Liau">PMID: 35768504</ref>. SHOC2 depletion is being studied as a therapeutic approach for RAS-driven cancers due to large scale interactions of the subunits being made possible by SHOC2 <ref name="Kwon">PMID: 35831509</ref>. As shown in '''Figure 1 '''SHOC2 and PP1C first engage in binding with each other via an N-terminal <scene name='95/952695/Rvxf_motif/2'>RVXF Motif</scene> on SHOC2 that is complimentary to a binding sequence on PP1C. SHOC2 residues <scene name='95/952695/Shoc2_highlighted_residues/1'>V64 and F66</scene> embed in the complimentary region of PP1C, enhancing SHOC2 affinity for PP1C. SHOC2 binds MRAS-GTP through β strands of a LRR that interacts with a [https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Physical_Properties_of_Matter/Atomic_and_Molecular_Properties/Intermolecular_Forces/Hydrophobic_Interactions. hydrophonic] region of MRAS-GTP further stabilizing the complex<ref name="Kwon">PMID: 35831509</ref>. | ||
=== PP1C === | === PP1C === | ||
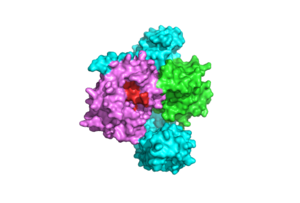
| - | <scene name='95/952695/Pp1cintro/3'>The Protein Phosphatase Complex 1 (PP1C)</scene> subunit contains the catalytic site of the SMP complex. PP1C is a [https://en.wikipedia.org/wiki/Phosphatase. Phosphatase] enzyme responsible for the removal of a phosphate group on the N-terminal phosphoserine (NTpS) of RAF (Ser259)<ref name="Liau">PMID: 35768504</ref>. The exact mechanism of dephosphorylation is currently unknown, but there are three catalytic metal ions: 2 Mn⁺² and 1 Cl⁻¹ present that coordinate nucleophilic water molecules in the active site <ref name="Hauseman">PMID:35830882</ref>. This dephosphorylation event allows for pathway activation <ref name="Liau">PMID: 35768504</ref>. Although PP1C can dephosphorylate other proteins independently from the SMP complex, it cannot act on RAF unless bound to the complex because it lacks intrinsic substrate selectivity <ref name="Liau">PMID: 35768504</ref>. SHOC2 and MRAS aid in the specificity of the enzymatic activity. PP1C binds to SHOC2 and MRAS-GTP in a specific orientation that doesn’t change the conformation of the catalytic site and leaves it accessible for substrate binding | + | [[Image:CS.png|300 px|right|thumb|'''Figure 2:''' Catalytic Site of PP1C (PDB 7DSO) <ref name="Liau">PMID: 35768504</ref>.]] |
| + | <scene name='95/952695/Pp1cintro/3'>The Protein Phosphatase Complex 1 (PP1C)</scene> subunit contains the catalytic site of the SMP complex. PP1C is a [https://en.wikipedia.org/wiki/Phosphatase. Phosphatase] enzyme responsible for the removal of a phosphate group on the N-terminal phosphoserine (NTpS) of RAF (Ser259)<ref name="Liau">PMID: 35768504</ref>. The exact mechanism of dephosphorylation is currently unknown, but there are three catalytic metal ions: 2 Mn⁺² and 1 Cl⁻¹ present that coordinate nucleophilic water molecules in the active site <ref name="Hauseman">PMID:35830882</ref>. This dephosphorylation event allows for pathway activation <ref name="Liau">PMID: 35768504</ref>. Although PP1C can dephosphorylate other proteins independently from the SMP complex, it cannot act on RAF unless bound to the complex because it lacks intrinsic substrate selectivity <ref name="Liau">PMID: 35768504</ref>. SHOC2 and MRAS aid in the specificity of the enzymatic activity. PP1C binds to SHOC2 and MRAS-GTP in a specific orientation that doesn’t change the conformation of the catalytic site and leaves it accessible for substrate binding as shown in '''Figure 2'''. | ||
PP1C binds to SHOC2 through a hydrophobic N-terminal disordered region that is complimentary to the <scene name='95/952695/Rvxf_motif/2'>RVXF Motif on SHOC2</scene> and adjacent to a catalytic metal ions <ref name="Liau">PMID: 35768504</ref>. In the RAS/RAF signaling cascade, the region of RAF that is C-terminal to the phosphate group binds to this hydrophobic groove, and the remaining residues bind to the hydrophobic region of SHOC2 <ref name="Hauseman">PMID:35830882</ref>. RAF binding to this region of SHOC2 is what allows PP1C to be specific when in the SMP complex in comparison to PP1C on its own <ref name="Hauseman">PMID:35830882</ref>. Similarly to SHOC2, PP1C does not undergo a <scene name='95/952694/Pp1coverlay/4'>significant conformational change</scene> when SHOC2 and MRAS-GTP bind. The lack of conformational change shows that the structure of PP1C is not dependent on the SMP complex, but in order to act as a phosphatase it must be bound to the complex <ref name="Liau">PMID: 35768504</ref>. | PP1C binds to SHOC2 through a hydrophobic N-terminal disordered region that is complimentary to the <scene name='95/952695/Rvxf_motif/2'>RVXF Motif on SHOC2</scene> and adjacent to a catalytic metal ions <ref name="Liau">PMID: 35768504</ref>. In the RAS/RAF signaling cascade, the region of RAF that is C-terminal to the phosphate group binds to this hydrophobic groove, and the remaining residues bind to the hydrophobic region of SHOC2 <ref name="Hauseman">PMID:35830882</ref>. RAF binding to this region of SHOC2 is what allows PP1C to be specific when in the SMP complex in comparison to PP1C on its own <ref name="Hauseman">PMID:35830882</ref>. Similarly to SHOC2, PP1C does not undergo a <scene name='95/952694/Pp1coverlay/4'>significant conformational change</scene> when SHOC2 and MRAS-GTP bind. The lack of conformational change shows that the structure of PP1C is not dependent on the SMP complex, but in order to act as a phosphatase it must be bound to the complex <ref name="Liau">PMID: 35768504</ref>. | ||
Revision as of 02:52, 17 April 2023
| This Sandbox is Reserved from February 27 through August 31, 2023 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1765 through Sandbox Reserved 1795. |
To get started:
More help: Help:Editing |
Contents |
SHOC2-PP1C-MRAS
| |||||||||||
Protopedia Resources
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 Kwon JJ, Hajian B, Bian Y, Young LC, Amor AJ, Fuller JR, Fraley CV, Sykes AM, So J, Pan J, Baker L, Lee SJ, Wheeler DB, Mayhew DL, Persky NS, Yang X, Root DE, Barsotti AM, Stamford AW, Perry CK, Burgin A, McCormick F, Lemke CT, Hahn WC, Aguirre AJ. Structure-function analysis of the SHOC2-MRAS-PP1C holophosphatase complex. Nature. 2022 Jul 13. pii: 10.1038/s41586-022-04928-2. doi:, 10.1038/s41586-022-04928-2. PMID:35831509 doi:http://dx.doi.org/10.1038/s41586-022-04928-2
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 Hauseman ZJ, Fodor M, Dhembi A, Viscomi J, Egli D, Bleu M, Katz S, Park E, Jang DM, Porter KA, Meili F, Guo H, Kerr G, Molle S, Velez-Vega C, Beyer KS, Galli GG, Maira SM, Stams T, Clark K, Eck MJ, Tordella L, Thoma CR, King DA. Structure of the MRAS-SHOC2-PP1C phosphatase complex. Nature. 2022 Jul 13. pii: 10.1038/s41586-022-05086-1. doi:, 10.1038/s41586-022-05086-1. PMID:35830882 doi:http://dx.doi.org/10.1038/s41586-022-05086-1
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 3.14 3.15 3.16 3.17 3.18 3.19 3.20 3.21 3.22 3.23 Liau NPD, Johnson MC, Izadi S, Gerosa L, Hammel M, Bruning JM, Wendorff TJ, Phung W, Hymowitz SG, Sudhamsu J. Structural basis for SHOC2 modulation of RAS signalling. Nature. 2022 Jun 29. pii: 10.1038/s41586-022-04838-3. doi:, 10.1038/s41586-022-04838-3. PMID:35768504 doi:http://dx.doi.org/10.1038/s41586-022-04838-3
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 Lavoie H, Therrien M. Structural keys unlock RAS-MAPK cellular signalling pathway. Nature. 2022 Sep;609(7926):248-249. PMID:35970881 doi:10.1038/d41586-022-02189-7
- ↑ 5.0 5.1 Young LC, Hartig N, Boned Del Río I, Sari S, Ringham-Terry B, Wainwright JR, Jones GG, McCormick F, Rodriguez-Viciana P. SHOC2-MRAS-PP1 complex positively regulates RAF activity and contributes to Noonan syndrome pathogenesis. Proc Natl Acad Sci U S A. 2018 Nov 6;115(45):E10576-E10585. PMID:30348783 doi:10.1073/pnas.1720352115
- ↑ Kelker MS, Page R, Peti W. Crystal structures of protein phosphatase-1 bound to nodularin-R and tautomycin: a novel scaffold for structure-based drug design of serine/threonine phosphatase inhibitors. J Mol Biol. 2009 Jan 9;385(1):11-21. Epub 2008 Nov 1. PMID:18992256 doi:10.1016/j.jmb.2008.10.053
Student Contributors
- Sloan August
- Rosa Trippel
- Kayla Wilhoite