We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1791
From Proteopedia
(Difference between revisions)
| Line 23: | Line 23: | ||
== Active vs. Inactive State == | == Active vs. Inactive State == | ||
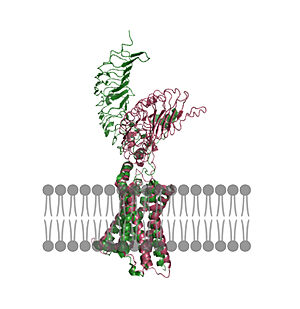
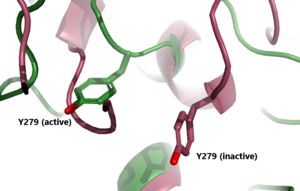
| - | In its resting state without TSH bound, TSHR is in the <scene name='95/952720/Inactivetshr/8'>inactive state</scene>, also known as the "down" state because the LRRD is pointing down. When TSH binds to TSHR, steric clashes between TSH and the cell-membrane cause TSHR to take on the <scene name='95/952720/Inactivetshr/6'>active or "up" state</scene> ( | + | In its resting state without TSH bound, TSHR is in the <scene name='95/952720/Inactivetshr/8'>inactive state</scene>, also known as the "down" state because the LRRD is pointing down. When TSH binds to TSHR, steric clashes between TSH and the cell-membrane cause TSHR to take on the <scene name='95/952720/Inactivetshr/6'>active or "up" state</scene> (Fig 2). During this transition, the LRRD rotate 55° along an axis perpendicular to the cell membrane. This rotation is initiated by conformational changes within the <scene name='95/952719/Hinge_region_spin/3'>Higne Region</scene>, specifically at the <scene name='95/952720/Hinge_region_residues/3'>Tyr279 residue</scene>, located in the Hinge Region. Y279 moves 6 Å relative to I486, which is a residue located in the Transmembrane Region nearby Y279<ref name="Faust"/> (Fig 3). |
The active form is favored when <scene name='95/952719/Active_form/6'>TSHR is bound to TSH</scene>. The structure can be seen as straight. The same straight conformation is observed when TSHR is bound with M22. The inactive form is found when <scene name='95/952719/Inactive_form/7'>TSHR is bound with K1</scene> of TSHR is found when bound with K1. The overall structure of the molecule is bent when K1 is bound. | The active form is favored when <scene name='95/952719/Active_form/6'>TSHR is bound to TSH</scene>. The structure can be seen as straight. The same straight conformation is observed when TSHR is bound with M22. The inactive form is found when <scene name='95/952719/Inactive_form/7'>TSHR is bound with K1</scene> of TSHR is found when bound with K1. The overall structure of the molecule is bent when K1 is bound. | ||
Revision as of 14:42, 21 April 2023
| This Sandbox is Reserved from February 27 through August 31, 2023 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1765 through Sandbox Reserved 1795. |
To get started:
More help: Help:Editing |
Thyroid Stimulating Hormone Receptor (TSHR)
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Faust B, Billesbolle CB, Suomivuori CM, Singh I, Zhang K, Hoppe N, Pinto AFM, Diedrich JK, Muftuoglu Y, Szkudlinski MW, Saghatelian A, Dror RO, Cheng Y, Manglik A. Autoantibody mimicry of hormone action at the thyrotropin receptor. Nature. 2022 Aug 8. pii: 10.1038/s41586-022-05159-1. doi:, 10.1038/s41586-022-05159-1. PMID:35940205 doi:http://dx.doi.org/10.1038/s41586-022-05159-1
- ↑ 2.0 2.1 2.2 2.3 Duan J, Xu P, Luan X, Ji Y, He X, Song N, Yuan Q, Jin Y, Cheng X, Jiang H, Zheng J, Zhang S, Jiang Y, Xu HE. Hormone- and antibody-mediated activation of the thyrotropin receptor. Nature. 2022 Aug 8. pii: 10.1038/s41586-022-05173-3. doi:, 10.1038/s41586-022-05173-3. PMID:35940204 doi:http://dx.doi.org/10.1038/s41586-022-05173-3
- ↑ 3.0 3.1 Fokina, E.F., Shpakov, A.O. Thyroid-Stimulating Hormone Receptor: the Role in the Development of Thyroid Pathology and Its Correction. J Evol Biochem Phys 58, 1439–1454 (2022). [DOI:10.1134/S0022093022050143 https://doi.org/10.1134/S0022093022050143]
- ↑ Chen CR, McLachlan SM, Rapoport B. Thyrotropin (TSH) receptor residue E251 in the extracellular leucine-rich repeat domain is critical for linking TSH binding to receptor activation. Endocrinology. 2010 Apr;151(4):1940-7. doi: 10.1210/en.2009-1430. Epub 2010 Feb 24. PMID: 20181794; PMCID: PMC2851189. [DOI 10.1210/en.2009-1430 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2851189/]
- ↑ 5.0 5.1 Goel R, Raju R, Maharudraiah J, Sameer Kumar GS, Ghosh K, Kumar A, Lakshmi TP, Sharma J, Sharma R, Balakrishnan L, Pan A, Kandasamy K, Christopher R, Krishna V, Mohan SS, Harsha HC, Mathur PP, Pandey A, Keshava Prasad TS. A Signaling Network of Thyroid-Stimulating Hormone. J Proteomics Bioinform. 2011 Oct 29;4:10.4172/jpb.1000195. PMID:24255551 doi:10.4172/jpb.1000195
- ↑ Maeda S, Koehl A, Matile H, Hu H, Hilger D, Schertler GFX, Manglik A, Skiniotis G, Dawson RJP, Kobilka BK. Development of an antibody fragment that stabilizes GPCR/G-protein complexes. Nat Commun. 2018 Sep 13;9(1):3712. doi: 10.1038/s41467-018-06002-w. PMID:30213947 doi:http://dx.doi.org/10.1038/s41467-018-06002-w
- ↑ 7.0 7.1 Nunez Miguel R, Sanders J, Chirgadze DY, Furmaniak J, Rees Smith B. Thyroid stimulating autoantibody M22 mimics TSH binding to the TSH receptor leucine rich domain: a comparative structural study of protein-protein interactions. J Mol Endocrinol. 2009 May;42(5):381-95. Epub 2009 Feb 16. PMID:19221175 doi:10.1677/JME-08-0152
- ↑ Furmaniak J, Sanders J, Sanders P, Li Y, Rees Smith B. TSH receptor specific monoclonal autoantibody K1-70TM targeting of the TSH receptor in subjects with Graves' disease and Graves' orbitopathy-Results from a phase I clinical trial. Clin Endocrinol (Oxf). 2022 Jun;96(6):878-887. doi: 10.1111/cen.14681. Epub 2022 Feb 6. PMID: 35088429; PMCID: PMC9305464. [DOI 10.1111/cen.14681]
- ↑ Smits G, Govaerts C, Nubourgh I, Pardo L, Vassart G, Costagliola S. Lysine 183 and glutamic acid 157 of the TSH receptor: two interacting residues with a key role in determining specificity toward TSH and human CG. Mol Endocrinol. 2002 Apr;16(4):722-35. doi: 10.1210/mend.16.4.0815. PMID: 11923469. [DOI: 10.1210/mend.16.4.0815 https://pubmed.ncbi.nlm.nih.gov/11923469/]
- ↑ 10.0 10.1 Chiovato L, Magri F, Carlé A. Hypothyroidism in Context: Where We've Been and Where We're Going. Adv Ther. 2019 Sep;36(Suppl 2):47-58. doi: 10.1007/s12325-019-01080-8. Epub 2019 Sep 4. PMID: 31485975; PMCID: PMC6822815. [DOI: 10.1007/s12325-019-01080-8 https://pubmed.ncbi.nlm.nih.gov/31485975/]
![Figure 1: An overview of the Thyroid System. A depiction of signaling cascade from the hypothalamus ending in the release of TSH causing T3 and T4 production and its effects. The mechanism of regulation is also shown by negative feedback from the T3 and T4 hormones. Source: [1]](/wiki/images/thumb/1/1d/TSH_system1.png/300px-TSH_system1.png)
![Figure 4: T3 and T4 role in TSH concentration: Highlighting the problem when under or overactive on the metabolism. When an antibody is bound to TSHR and cannot respond to the negative feedback look the metabolism experiences a shift outside of equilibrium resulting in a wide array of side effects. [2]](/wiki/images/thumb/5/5f/T3t4levels.jpeg/400px-T3t4levels.jpeg)
