User:João Pedro de Carvalho Pereira/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 25: | Line 25: | ||
<br> | <br> | ||
Within the larger domain, more specifically in the active <scene name='97/973103/Ligacao_plp/1'>binding site</scene> for PLP, the amino acid residues that effectively engage with this ligand are situated in the central region of the beta sheet and in close proximity to adjacent alpha helices. A total of six amino acids establish hydrogen bond interactions (Gly<sup>90</sup>, Leu<sup>91</sup>, Asp<sup>187</sup>, Ser<sup>209</sup>, Thr<sup>211</sup>, and Lys<sup>212</sup>). Moreover, two amino acids from the neighboring subunit also establish hydrogen bond interactions with PLP(Tyr<sup>60</sup> and Arg<sup>62</sup>)<ref name="x"/>.Another significant aspect is that the amino acid Tyr<sup>114</sup> appears to hold great relevance in enzymatic activity, as it interacts with the pyrimidine ring of PLP through aromatic stacking.<br> | Within the larger domain, more specifically in the active <scene name='97/973103/Ligacao_plp/1'>binding site</scene> for PLP, the amino acid residues that effectively engage with this ligand are situated in the central region of the beta sheet and in close proximity to adjacent alpha helices. A total of six amino acids establish hydrogen bond interactions (Gly<sup>90</sup>, Leu<sup>91</sup>, Asp<sup>187</sup>, Ser<sup>209</sup>, Thr<sup>211</sup>, and Lys<sup>212</sup>). Moreover, two amino acids from the neighboring subunit also establish hydrogen bond interactions with PLP(Tyr<sup>60</sup> and Arg<sup>62</sup>)<ref name="x"/>.Another significant aspect is that the amino acid Tyr<sup>114</sup> appears to hold great relevance in enzymatic activity, as it interacts with the pyrimidine ring of PLP through aromatic stacking.<br> | ||
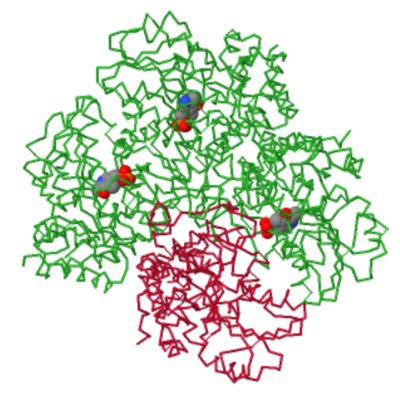
| - | Upon juxtaposing the structure of cystathionine gamma-lyase in the absence of the cofactor and its bound state, notable distinctions in the configuration of its active site come to light. In the unbound state, the active site displays a discernibly more unobstructed conformation, whereas its association with PLP induces a marked closure. The most pronounced alterations manifest within <scene name='97/973103/Sitio_ativo_fechado/1'>two distinct loop regions</scene> (amino acids Met<sup>110</sup>-Asn<sup>118</sup> and Thr<sup>210</sup>-Met<sup>216</sup>)<ref name="x"/>. A noteworthy characteristic regarding the conformational changes of this protein is that, in its PLP-bound state, the tetrameric asymmetric unit encompasses one of its subunits lacking the presence of the cofactor (with only 3 subunits remaining bound). This observation suggests that the enzyme exhibits substantial dynamism and is likely to exist as an amalgamation of various conformational states<ref name="x"/>. | + | Upon juxtaposing the structure of cystathionine gamma-lyase in the absence of the cofactor and its bound state, notable distinctions in the configuration of its active site come to light. In the unbound state, the active site displays a discernibly more unobstructed conformation, whereas its association with PLP induces a marked closure. The most pronounced alterations manifest within <scene name='97/973103/Sitio_ativo_fechado/1'>two distinct loop regions</scene> (amino acids Met<sup>110</sup>-Asn<sup>118</sup> and Thr<sup>210</sup>-Met<sup>216</sup>)<ref name="x"/>. A noteworthy characteristic regarding the conformational changes of this protein is that, in its PLP-bound state, the tetrameric asymmetric unit encompasses one of its subunits lacking the presence of the cofactor (with only 3 subunits remaining bound). This observation suggests that the enzyme exhibits substantial dynamism and is likely to exist as an amalgamation of various conformational states<ref name="x"/>. |
| + | [[Image:Dominio vazio.png|400px|center|thumb| The three subunits that bind to PLP are depicted in green, while the empty unit is shown in red. Image generatade with JSmol.]] | ||
| + | |||
Revision as of 15:52, 25 June 2023
Cystathionine gamma-lyase (Homo sapiens)
| |||||||||||
References
- ↑ 1.0 1.1 Chiku T, Padovani D, Zhu W, Singh S, Vitvitsky V, Banerjee R. H2S biogenesis by human cystathionine gamma-lyase leads to the novel sulfur metabolites lanthionine and homolanthionine and is responsive to the grade of hyperhomocysteinemia. J Biol Chem. 2009 Apr 24;284(17):11601-12. doi: 10.1074/jbc.M808026200. Epub 2009, Mar 4. PMID:19261609 doi:10.1074/jbc.M808026200
- ↑ Messerschmidt A, Worbs M, Steegborn C, Wahl MC, Huber R, Laber B, Clausen T. Determinants of enzymatic specificity in the Cys-Met-metabolism PLP-dependent enzymes family: crystal structure of cystathionine gamma-lyase from yeast and intrafamiliar structure comparison. Biol Chem. 2003 Mar;384(3):373-86. PMID:12715888
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 Sun Q, Collins R, Huang S, Holmberg-Schiavone L, Anand GS, Tan CH, van-den-Berg S, Deng LW, Moore PK, Karlberg T, Sivaraman J. Structural basis for the inhibition mechanism of human cystathionine gamma-lyase, an enzyme responsible for the production of H(2)S. J Biol Chem. 2009 Jan 30;284(5):3076-85. Epub 2008 Nov 19. PMID:19019829 doi:http://dx.doi.org/10.1074/jbc.M805459200
- ↑ Adaikan PG, Karim SM. Effects of PGA and PGB compounds on gastrointestinal tract smooth muscle from man and laboratory animals. Prostaglandins. 1976 Jan;11(1):15-22. PMID:1257494 doi:10.1016/0090-6980(76)90168-4
- ↑ Scott CR, Dassell SW, Clark SH, Chiang-Teng C, Swedberg KR. Cystathioninemia: a benign genetic condition. J Pediatr. 1970 Apr;76(4):571-7. PMID:5420794 doi:10.1016/s0022-3476(70)80407-3
- ↑ Wang J, Hegele RA. Genomic basis of cystathioninuria (MIM 219500) revealed by multiple mutations in cystathionine gamma-lyase (CTH). Hum Genet. 2003 Apr;112(4):404-8. Epub 2003 Feb 6. PMID:12574942 doi:10.1007/s00439-003-0906-8