User:Matheus Andrade Bettiol/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 9: | Line 9: | ||
'''3.''' GDI ([[Guanine nucleotide dissociation inhibitor]]): translocates the membrane GTPase, sequestering it to the cytosol, also inhibiting RhoA. | '''3.''' GDI ([[Guanine nucleotide dissociation inhibitor]]): translocates the membrane GTPase, sequestering it to the cytosol, also inhibiting RhoA. | ||
| - | This protein is an | + | This protein is an essential molecular switch for activities associated with the cytoskeleton and the immune system. In relation to the cytoskeleton, it is a regulator of cell migration, contributing to cell retraction through the ROCK ([[Rho-associated protein kinase]]) and LIMK (LIM kinase) pathway, which leads to contraction of actomyosin II and [[actin]] polymerization. In addition, it is relevant for the assembly of occlusive junctions that seal the epithelium in selective permeability barriers, such as in the intestine. When it comes to the immune system, it is essential for the presentation of antigens and formation of immune synapses between the dendritic cell and the T lymphocyte. Not only that, but it also contributes to the recruitment and phagocytosis activity of neutrophils, macrophages and dendritic cells <ref>PMID:31319592</ref>. |
| Line 38: | Line 38: | ||
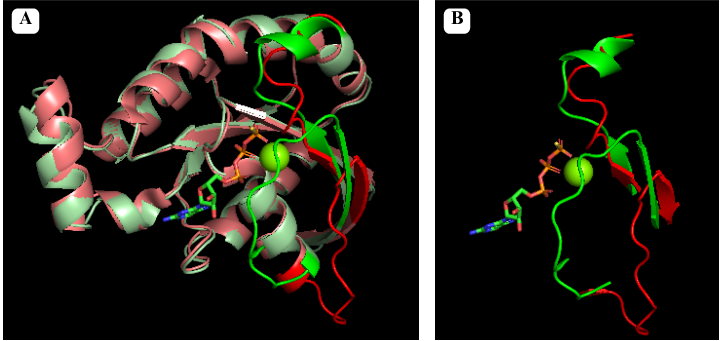
[[Image:A.png]] | [[Image:A.png]] | ||
| - | + | ''Pymol alignment of <span style="color:green;background-color:black;font-weight:bold;"> GTP bound RhoA in green</span> and <span style="color:red;background-color:black;font-weight:bold;">GDP bound RhoA in red</span>. Figure A shows the full alignment with Switch I and II represented with more saturated colors. Figure B shows just Switch I and II'' | |
<scene name='97/973102/Insertion_region/2'>Rho Insert</scene>: This is a unique sequence insertion found within the GTPase domain of RhoA. It plays a role in the regulation and interaction of RhoA with other proteins. | <scene name='97/973102/Insertion_region/2'>Rho Insert</scene>: This is a unique sequence insertion found within the GTPase domain of RhoA. It plays a role in the regulation and interaction of RhoA with other proteins. | ||
| Line 46: | Line 46: | ||
== Post-Translational Modifications == | == Post-Translational Modifications == | ||
| - | '''Prenylation:''' | + | '''Prenylation:''' The activation of [[Rho GTPase]] requires membrane binding, which is necessary for the interaction with membranous GEFs. The membrane association requires C-terminal prenylation, which involves the addition of a geranylgeranyl (20-carbon chain) to Cys190 in the CAAX motif. |
| - | '''Phosphorylation:''' | + | '''Phosphorylation:''' Can alter the subcellular localization of RhoA when occurs close to C-terminal lipid modifications. On the other hand, phosphorylation of the G-domain affects GTP/GDP cycling and the interaction with effector proteins. |
'''Oxidation:''' RhoA can be oxidized on Cys16 and Cys20 (G1 domain), generating a disulfide bond that prevents guanine binding and GEF association, inactivating RhoA. However, if Tyr42 is phosphorylated, serving as a binding site for GEF, oxidation on Cys16/20 reduces the affinity of RhoA for GDI and increases the association with GEF, leading to RhoA activation. | '''Oxidation:''' RhoA can be oxidized on Cys16 and Cys20 (G1 domain), generating a disulfide bond that prevents guanine binding and GEF association, inactivating RhoA. However, if Tyr42 is phosphorylated, serving as a binding site for GEF, oxidation on Cys16/20 reduces the affinity of RhoA for GDI and increases the association with GEF, leading to RhoA activation. | ||
| - | '''Nitration:''' | + | '''Nitration:''' Nitration on RhoA's Tyr34 (switch I region) introduces a negative charge that modifies the protein structure and leads to a faster GDP release and GTP reload, increasing RhoA activity. |
| - | '''Adenylation:''' | + | '''Adenylation:''' Adenylation on Tyr34 (switch I region) leads to RhoA inhibition. |
'''Ubiquitination''': target the protein for degradation by the proteasome. RhoA is ubiquitylated by E3 [[ubiquitin protein ligase]] complexes, that ubiquitinate either active RhoA on Lys6 and Lys7, inactive RhoA, or both states on Lys135.<ref>PMID:35563826</ref> | '''Ubiquitination''': target the protein for degradation by the proteasome. RhoA is ubiquitylated by E3 [[ubiquitin protein ligase]] complexes, that ubiquitinate either active RhoA on Lys6 and Lys7, inactive RhoA, or both states on Lys135.<ref>PMID:35563826</ref> | ||
Revision as of 20:20, 14 July 2023
| |||||||||||
References
- ↑ Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247-69. PMID:16212495 doi:10.1146/annurev.cellbio.21.020604.150721
- ↑ Bros M, Haas K, Moll L, Grabbe S. RhoA as a Key Regulator of Innate and Adaptive Immunity. Cells. 2019 Jul 17;8(7):733. PMID:31319592 doi:10.3390/cells8070733
- ↑ Hetmanski JH, Zindy E, Schwartz JM, Caswell PT. A MAPK-Driven Feedback Loop Suppresses Rac Activity to Promote RhoA-Driven Cancer Cell Invasion. PLoS Comput Biol. 2016 May 3;12(5):e1004909. PMID:27138333 doi:10.1371/journal.pcbi.1004909
- ↑ Schmidt SI, Blaabjerg M, Freude K, Meyer M. RhoA Signaling in Neurodegenerative Diseases. Cells. 2022 May 1;11(9):1520. PMID:35563826 doi:10.3390/cells11091520
- ↑ Xu H, Yang J, Gao W, Li L, Li P, Zhang L, Gong YN, Peng X, Xi JJ, Chen S, Wang F, Shao F. Innate immune sensing of bacterial modifications of Rho GTPases by the Pyrin inflammasome. Nature. 2014 Sep 11;513(7517):237-41. doi: 10.1038/nature13449. Epub 2014 Jun 11. PMID:24919149 doi:http://dx.doi.org/10.1038/nature13449
- ↑ Ihara K, Muraguchi S, Kato M, Shimizu T, Shirakawa M, Kuroda S, Kaibuchi K, Hakoshima T. Crystal structure of human RhoA in a dominantly active form complexed with a GTP analogue. J Biol Chem. 1998 Apr 17;273(16):9656-66. PMID:9545299
- ↑ Shimizu T, Ihara K, Maesaki R, Kuroda S, Kaibuchi K, Hakoshima T. An open conformation of switch I revealed by the crystal structure of a Mg2+-free form of RHOA complexed with GDP. Implications for the GDP/GTP exchange mechanism. J Biol Chem. 2000 Jun 16;275(24):18311-7. PMID:10748207 doi:10.1074/jbc.M910274199
- ↑ Schmidt SI, Blaabjerg M, Freude K, Meyer M. RhoA Signaling in Neurodegenerative Diseases. Cells. 2022 May 1;11(9):1520. PMID:35563826 doi:10.3390/cells11091520