User:Jordan RG Elliott/Sandbox 1
From Proteopedia
| Line 19: | Line 19: | ||
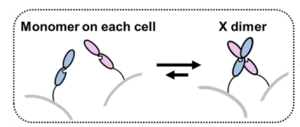
| - | The crystal structure 3K5S (2.90 Å resolution) encompasses two extracellular cadherin domains (EC1 and EC2) of chick T-cadherin forming a symmetric <scene name='10/1079500/X-dimer_structure_highlight/1'>X-shaped dimer</scene>, contacting each other at the center of the cross. The individual extracellular units consist of 2 β-barrel sections separated by the interdomain area of hydrophobic residues <scene name='10/1079500/Middle_section_binding/1'>(Met198, Val203, Leu205)</scene>(Blue EC1, green EC2, Spheres Ca 2+) where the Ca 2+ ligand ions and to the other extracellular unit are bound to. The truncated C terminus is visible at the bottom of each unit, pointing towards the cell the EC is attached to, this is where the intermembrane domain would have protruded from in a classical cadherin. Instead, the anchoring method is added post-translationally in the form of a GPI anchor made to fit the C-terminus region where a hydrophobic sequence of amino acids directs the attachment of the GPI anchor. | + | The crystal structure 3K5S (2.90 Å resolution) encompasses two extracellular cadherin domains (EC1 and EC2) of chick T-cadherin forming a symmetric <scene name='10/1079500/X-dimer_structure_highlight/1'>X-shaped dimer</scene>, contacting each other at the center of the cross. The individual extracellular units consist of 2 β-barrel sections separated by the interdomain area of hydrophobic residues <scene name='10/1079500/Middle_section_binding/1'>(Met198, Val203, Leu205)</scene>(Blue EC1, green EC2, Spheres Ca 2+) where the Ca 2+ ligand ions and to the other extracellular unit are bound to. The <scene name='10/1079500/Truncated_c_term/1'>truncated C terminus</scene> is visible at the bottom of each unit, pointing towards the cell the EC is attached to, this is where the intermembrane domain would have protruded from in a classical cadherin. Instead, the anchoring method is added post-translationally in the form of a GPI anchor made to fit the C-terminus region where a hydrophobic sequence of amino acids directs the attachment of the GPI anchor. |
[[Image:X-Dimer T-cadherin.png|thumb|right|300px|Simple Demonstration of X-dimer binding]] | [[Image:X-Dimer T-cadherin.png|thumb|right|300px|Simple Demonstration of X-dimer binding]] | ||
Revision as of 23:16, 30 April 2025
Contents |
Crystal structure of chicken T-cadherin EC1 EC2
Introduction
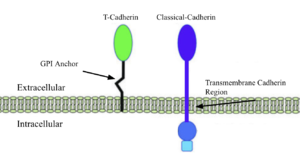
The Cadherins are membrane proteins that mediate cell–cell adhesion and are essential for the development and structural integrity of tissues at the cellular level. make up much of the cadherin family, such as E-, N-, and P-cadherins, which facilitate homophilic adhesion through a mechanism known as “strand swapping,” in which the N-terminal β-strands of cadherin molecules from opposing cells are exchanged. (Gumbiner, B. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol 6, 622–634 (2005). https://doi.org/10.1038/nrm1699). Classical cadherins and their strand swapping are the standard for many organisms because of their adhesion strength and stability due to their membrane integration and low extracellular motility.
T-Cadherins, truncated cadherins, are a sect of nonclassical cadherins, unique molecules that distinguish themselves from the above with different membrane anchorage techniques and functions, while retaining similar motifs and structures. Strand swapping and membrane integration, the two aspects of cadherins that allow them to provide structure and communication, are absent in T-cadherins as they lack the for strand swapping and the hydrophobic a-helix for membrane integration.
Function
The 3K5S T-cadherin is attached peripherally to the cell surface, via a GPI anchor, having no inner membrane or cytoplasmic domain, shortening the total weight and length of a single unit, hence the name truncated. (Ranscht B, Dours-Zimmermann MT. T-cadherin, a novel cadherin cell adhesion molecule in the nervous system, lacks the conserved cytoplasmic region. Neuron. 1991 Sep;7(3):391-402. doi: 10.1016/0896-6273(91)90291-7. PMID: 1654948.) This binding method and reduced size benefit 3K5S in its role of aiding neurite development and signaling in chick growth. The soft structure and shorter signalling reach allows for a more fluid and less tense growth environment for neural development. The concentration of 3K5S is slowly diminished as other cadherins, because more necessary for adult synaptic maintenance. (https://www.sciencedirect.com/science/article/pii/S0006899308002813#:~:text=Three%20types%20of%20full%2Dlength%20cDNAs%20encoding%20chicken,high%20similarity%20to%20rat%20and%20human%20Cdh8.)
Structural Highlights
| |||||||||||