User:Sheetal Patil
From Proteopedia
| Line 1: | Line 1: | ||
| - | <code></code>I | + | <code></code>I am an undergraduate student at the University of Maryland Baltimore County and a biocehmistry project we are working on is on the Glutamine Synthetase enzyme. |
| + | |||
<applet load='2qc8' size='300' frame='true' align='right' /> | <applet load='2qc8' size='300' frame='true' align='right' /> | ||
| Line 20: | Line 21: | ||
| - | + | Explanation: | |
| Line 35: | Line 36: | ||
Studies in competitive inhibition of glutamine synthetase show that glycine, | Studies in competitive inhibition of glutamine synthetase show that glycine, | ||
L-alanine, and L-serine are all inhibitors competing for the same binding site for | L-alanine, and L-serine are all inhibitors competing for the same binding site for | ||
| - | L-glutamate. They also competing to bind to GS-ATP. These sites can be seen on the | + | L-glutamate. They are also competing to bind to GS-ATP. These sites can be seen on the |
molecule here. (Insert wiki text) L-alanine specifically does not show any affinity | molecule here. (Insert wiki text) L-alanine specifically does not show any affinity | ||
to GS that is substantially lower than that of glutamate. Also, no significant | to GS that is substantially lower than that of glutamate. Also, no significant | ||
conformation change is shown by the binding of these competitive inhibitors other | conformation change is shown by the binding of these competitive inhibitors other | ||
than the movement of Asn-264. | than the movement of Asn-264. | ||
Revision as of 02:24, 11 December 2008
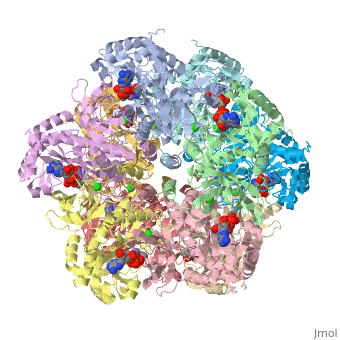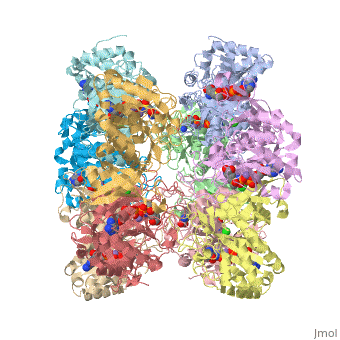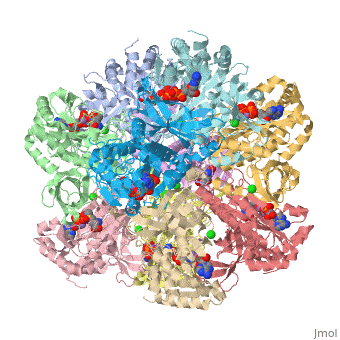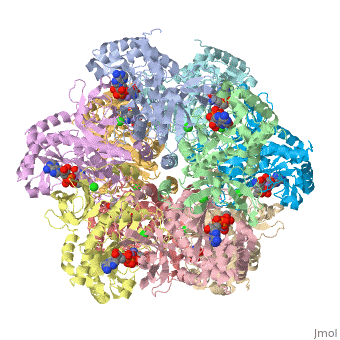
I am an undergraduate student at the University of Maryland Baltimore County and a biocehmistry project we are working on is on the Glutamine Synthetase enzyme.
|
Exercise 1
Exercise 2
Exercise 3
Exercise 4
Explanation:
Competitive inhibition takes place when a molecule that is structurally similar
to the substrate for a particular reaction competes for its position at the active site on the enzyme. Sites that ligands are allowed to bind can be shown here. This ties up the enzyme so that it is not available to the substrate. Competitive inhibition can be reversed if the concentration of substrate is raised to sufficiently high levels while the concentration of the inhibitor is held constant.
Studies in competitive inhibition of glutamine synthetase show that glycine,
L-alanine, and L-serine are all inhibitors competing for the same binding site for L-glutamate. They are also competing to bind to GS-ATP. These sites can be seen on the molecule here. (Insert wiki text) L-alanine specifically does not show any affinity to GS that is substantially lower than that of glutamate. Also, no significant conformation change is shown by the binding of these competitive inhibitors other than the movement of Asn-264.