Prion protein
From Proteopedia
| Line 1: | Line 1: | ||
| - | The prion protein (PrP) is a cell surface glycoprotein, which can exist in two alternatively folded | + | The prion protein (PrP) is a cell surface glycoprotein, which can exist in two alternatively folded conformations: a cellular isoform denoted (PrP<sup>C</sup>) and a disease associated isoform termed PrP<sup>Sc</sup>. |
=Prion diseases= | =Prion diseases= | ||
| - | The naturally ocuring prion diseases include Creutzfeldt Jakob disease (CJD) in people, bovine spongiform encephalopathy (BSE) commonly known as "mad cow" disease, | + | The naturally ocuring prion diseases include Creutzfeldt Jakob disease (CJD) in people, bovine spongiform encephalopathy (BSE) commonly known as "mad cow" disease, scrapie in sheep and goats, and chronic wasting disease in cervids. ''Post mortem'' analysis of brain tissue is characterterized by aggregates of PrP<sup>Sc</sup>. |
| - | The spontaneous, genetic and infectious etiologies of prion diseases can be explained by a simple protein-based model in which PrP<sup>C</sup> is converted into PrP<sup>Sc</sup> | + | The spontaneous, genetic and infectious etiologies of prion diseases can be explained by a simple protein-based model in which PrP<sup>C</sup> is converted into PrP<sup>Sc</sup> that initiates a cascade of autocatalytic refolding of PrP<sup>C</sup> in a template-dependent manner. |
| - | In sporadic disease, the spontaneous refolding or misfolding of PrP<sup>C</sup> into PrP<sup>Sc</sup> initiates the cascade. In genetic prion diseases, point mutations in PrP make this more likely to happen than in the wild type protein, Infectious etiology is explained by introduction of exogenous PrP<sup>Sc</sup> which then | + | In sporadic disease, the spontaneous refolding or misfolding of PrP<sup>C</sup> into PrP<sup>Sc</sup> initiates the cascade. In genetic prion diseases, point mutations in PrP make this structural transition more likely to happen than in the wild type protein, Infectious etiology is explained by introduction of exogenous PrP<sup>Sc</sup> which then initiates refolding of endogenous PrP<sup>C</sup>. |
=Structure of PrP<sup>C</sup>= | =Structure of PrP<sup>C</sup>= | ||
{{STRUCTURE_1hjm | PDB=1hjm | SCENE= }} | {{STRUCTURE_1hjm | PDB=1hjm | SCENE= }} | ||
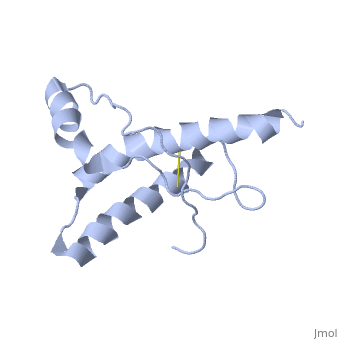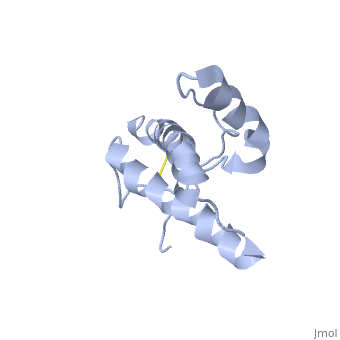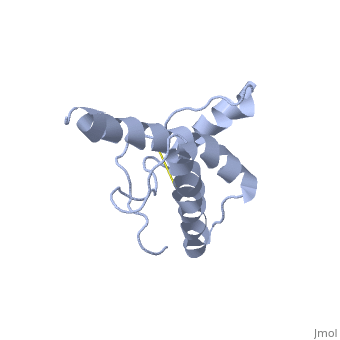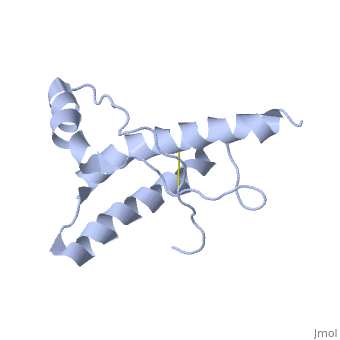
| - | PrP<sup>C</sup> has a natively unstructured N-terminal region, and a predominantly α-helical C-terminal region from residues ~120-230 | + | PrP<sup>C</sup> has a natively unstructured N-terminal region, and a predominantly α-helical C-terminal region from residues ~120-230. containing three α-helices and two short β-strands. A single disulfide bond connects the middle of helices 2 and 3. The presence of the N-terminal region has little impact on the structure of the C-terminal domain <ref>Zahn, R. ''et al.'' (2000) NMR solution structure of the human prion protein ''Proc. Natl. Acad. Sci. USA'' '''97''', 145-150 </ref>. |
| - | + | , and the structure of PrP<sup>C</sup> is highly conserved amongst mammals, and only differs slightly in birds, reptiles and amphibians<ref>[[Pan, KM ''et al.'' (1993) Conversion of alpha-helices into beta-sheets features in the formation of the scrapie prion proteins ''Proc. Natl. Acad. Sci. USA'' '''90''', 10962-10966}}</ref>. | |
| - | The vast majority of structures have been determined by | + | The vast majority of structures have been determined by NMR spectroscopy, but two structures have been reported by X-ray crystallography. In sheep PrP, the structure is similar to other PrPs determined by NMR spectroscopy, however in human PrP, the X-ray structure is a dimer in which helix 3 is swapped with respect to the monomer and the disulphide bond is rearranged to be intermolecular between the dimer subunits. |
| - | Although having a similar overall fold, the X-ray structure of sheep PrP was dimeric | ||
=Models of PrP<sup>Sc</sup> structure= | =Models of PrP<sup>Sc</sup> structure= | ||
| - | + | Fourier transform infrared (FTIR) spectroscopy, and circular dichroism (CD) studies first demonstrated that PrP<sup>Sc</sup> had very different proportions of α-helices and β-sheet to PrP<sup>C</sup><ref>{{Calzolai, L ''et al.'' (2005) Prion protein NMR structures of chicken, turtle, and frog 'Proc. Natl. Acad. Sci. USA'' '''102''', 651-655}}</ref>. | |
| - | + | There are a number of technical obstacles in determining the molecular structure of PrP(sup)Sc</sup>, and the highest resolution structural information to date has been obtained by electron microscopy of 2D crystals. Differential binding of metal ions to these 2D crystals, and redacted constructs of PrP, provide a basis for structural modeling. | |
| - | + | A model the N-terminal region and much of the C-terminal domain, up to the disulphide bond, refolds into a β-helical structure | |
| - | + | Support for this β-helical model comes from the structure of the fungal prion Het-s ([[2rnm]]). | |
| - | There are a number of technical obstacles in determining the molecular structure of PrP | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
=Prion strains= | =Prion strains= | ||
| - | The strain phenomenon of | + | The strain phenomenon of prion strains (disease subtype replicating with high fidelity and producing specific clinical, biochemical and neuropathological features) was initially difficult to equate with the "protein only" hypothesis of prion diseases. However, there is now evidence from a range if studies suggesting that strains are enciphered in the structure of PrP<sup>Sc</sup>. One potential mechanism for this is alternate threading of the β-helix. |
| - | + | ||
=Selected PrP structures= | =Selected PrP structures= | ||
Revision as of 15:59, 14 December 2008
The prion protein (PrP) is a cell surface glycoprotein, which can exist in two alternatively folded conformations: a cellular isoform denoted (PrPC) and a disease associated isoform termed PrPSc.
Contents |
Prion diseases
The naturally ocuring prion diseases include Creutzfeldt Jakob disease (CJD) in people, bovine spongiform encephalopathy (BSE) commonly known as "mad cow" disease, scrapie in sheep and goats, and chronic wasting disease in cervids. Post mortem analysis of brain tissue is characterterized by aggregates of PrPSc. The spontaneous, genetic and infectious etiologies of prion diseases can be explained by a simple protein-based model in which PrPC is converted into PrPSc that initiates a cascade of autocatalytic refolding of PrPC in a template-dependent manner.
In sporadic disease, the spontaneous refolding or misfolding of PrPC into PrPSc initiates the cascade. In genetic prion diseases, point mutations in PrP make this structural transition more likely to happen than in the wild type protein, Infectious etiology is explained by introduction of exogenous PrPSc which then initiates refolding of endogenous PrPC.
Structure of PrPC
| |||||||||
| 1hjm, 1 NMR models () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Related: | 1e1g, 1e1j, 1e1p, 1e1s, 1e1u, 1e1w, 1hjn | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
PrPC has a natively unstructured N-terminal region, and a predominantly α-helical C-terminal region from residues ~120-230. containing three α-helices and two short β-strands. A single disulfide bond connects the middle of helices 2 and 3. The presence of the N-terminal region has little impact on the structure of the C-terminal domain [1].
, and the structure of PrPC is highly conserved amongst mammals, and only differs slightly in birds, reptiles and amphibians[2]. The vast majority of structures have been determined by NMR spectroscopy, but two structures have been reported by X-ray crystallography. In sheep PrP, the structure is similar to other PrPs determined by NMR spectroscopy, however in human PrP, the X-ray structure is a dimer in which helix 3 is swapped with respect to the monomer and the disulphide bond is rearranged to be intermolecular between the dimer subunits.
Models of PrPSc structure
Fourier transform infrared (FTIR) spectroscopy, and circular dichroism (CD) studies first demonstrated that PrPSc had very different proportions of α-helices and β-sheet to PrPC[3]. There are a number of technical obstacles in determining the molecular structure of PrP(sup)Sc</sup>, and the highest resolution structural information to date has been obtained by electron microscopy of 2D crystals. Differential binding of metal ions to these 2D crystals, and redacted constructs of PrP, provide a basis for structural modeling. A model the N-terminal region and much of the C-terminal domain, up to the disulphide bond, refolds into a β-helical structure Support for this β-helical model comes from the structure of the fungal prion Het-s (2rnm).
Prion strains
The strain phenomenon of prion strains (disease subtype replicating with high fidelity and producing specific clinical, biochemical and neuropathological features) was initially difficult to equate with the "protein only" hypothesis of prion diseases. However, there is now evidence from a range if studies suggesting that strains are enciphered in the structure of PrPSc. One potential mechanism for this is alternate threading of the β-helix.
Selected PrP structures
All structures determined by NMR unless otherwise specified
Human PrP
- 1qlx HuPrP residues 23-230
- 1qm0 HuPrP residues 90-230
- 1qm2 HuPrP residues 121-230
- 1i4m HuPrP residues 119-226 (determined by X-ray crystallography)
- 1fkc HuPrP,E200K residues 90-231 (genetic prion disease)
- 1h0l HuPrP residues 121-230, with an additional disulphide bond analogous to the homolog Doppel
Other species PrPs
- 1xyx Mouse PrP residues
- 1b10 Syrian hamster PrP residues 90-231
- 1dwy Cow PrP residues 121-230
- 1uw3 Sheep PrP (determined by X-ray crystallography)
- 1xu0 Frog PrP residues 98-226
- 1u3m Chicken PrP
- 1u5l Turtle PrP residues 121-226
References
- ↑ Zahn, R. et al. (2000) NMR solution structure of the human prion protein Proc. Natl. Acad. Sci. USA 97, 145-150
- ↑ [[Pan, KM et al. (1993) Conversion of alpha-helices into beta-sheets features in the formation of the scrapie prion proteins Proc. Natl. Acad. Sci. USA 90, 10962-10966}}
- ↑ [[:Template:Calzolai, L et al. (2005) Prion protein NMR structures of chicken, turtle, and frog 'Proc. Natl. Acad. Sci. USA 102, 651-655]]
Proteopedia Page Contributors and Editors (what is this?)
Kurt Giles, Joel L. Sussman, Jaime Prilusky, Michal Harel, Claudio Garutti, Eran Hodis