Proton Channels
From Proteopedia
| Line 10: | Line 10: | ||
caption='Linear interpolation morph from [http://proteopedia.org/wiki/index.php/3bkd 3bkd] to [http://proteopedia.org/wiki/index.php/2rlf 2rlf model 1] ' /> | caption='Linear interpolation morph from [http://proteopedia.org/wiki/index.php/3bkd 3bkd] to [http://proteopedia.org/wiki/index.php/2rlf 2rlf model 1] ' /> | ||
--> | --> | ||
| - | At right is a [[Morphs|linear-interpolation morph]] between 3BKD and 2RLF, showing the proposed opening and closing of this channel. | + | At right is a [[Morphs|linear-interpolation morph]] between 3BKD and 2RLF, showing the proposed opening and closing of this channel. {{Button_Toggle_Animation2}} |
In addition to watching the animation as alpha-helical ribbons, it is useful to watch it <scene name='Proton_Channels/Spacefilled/1'>spacefilled</scene>. '''Be sure to rotate the molecule with your mouse to watch the animation from different perspectives!''' | In addition to watching the animation as alpha-helical ribbons, it is useful to watch it <scene name='Proton_Channels/Spacefilled/1'>spacefilled</scene>. '''Be sure to rotate the molecule with your mouse to watch the animation from different perspectives!''' | ||
Revision as of 21:27, 9 March 2010
|
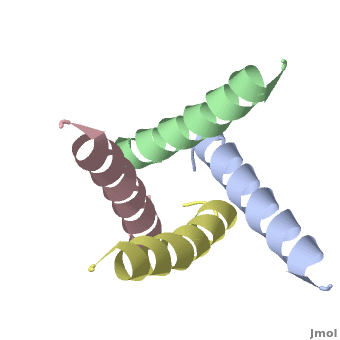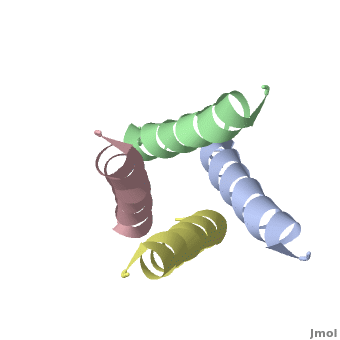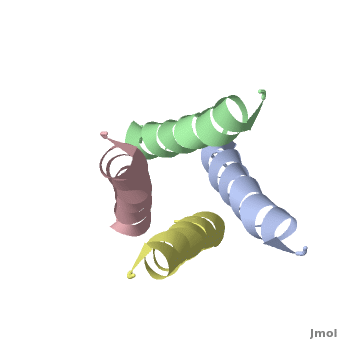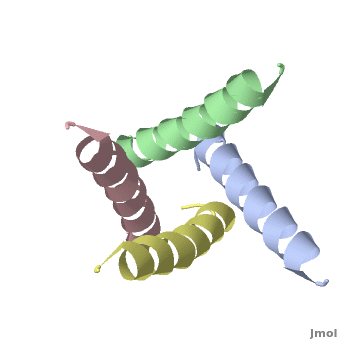
The M2 protein of influenza A virus is a proton channel. Its function is essential for productive infection by the virus.
See Category:Proton_channel for a list of all proton channel structures.
In January, 2008, crystallographic and NMR structures were published side by side in Nature for the transmembrane domains of the M2 protein: 3bkd to 2rlf. The former appeared to be in an open conformation blocked by amantadine, while the latter appeared to be in a closed conformation stabilized by rimantadine. (Neither drug is shown in the morph at right.)
At right is a linear-interpolation morph between 3BKD and 2RLF, showing the proposed opening and closing of this channel.
In addition to watching the animation as alpha-helical ribbons, it is useful to watch it . Be sure to rotate the molecule with your mouse to watch the animation from different perspectives!
are believed to be crucial for pH-dependent gating. (The apparent collapse and re-expansion of their sidechains is an artifact due to the linear interpolation method of morphing.) Here are His and Trp .
To be explained in a later revision, along with new scenes: Morph from Yale
Proteopedia Page Contributors and Editors (what is this?)
Eric Martz, Eran Hodis, David Canner, Joel L. Sussman, Michal Harel, Alexander Berchansky, Jaime Prilusky