We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Beta-2 Adrenergic Receptor
From Proteopedia
(Difference between revisions)
| Line 9: | Line 9: | ||
====Strucutre of B2ARs==== | ====Strucutre of B2ARs==== | ||
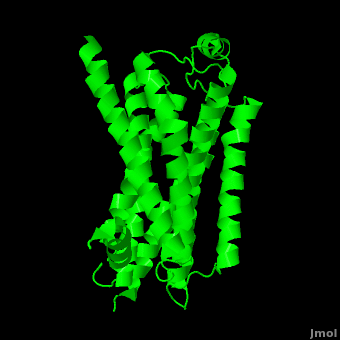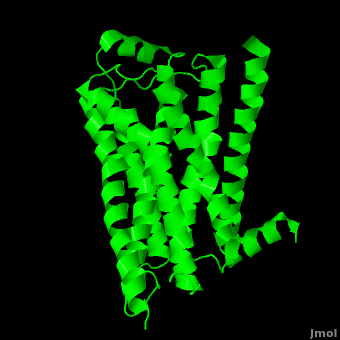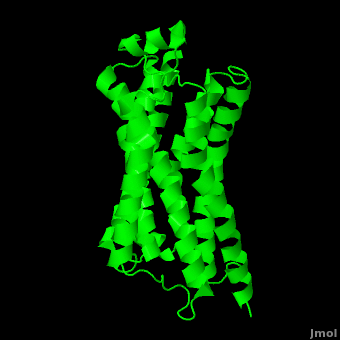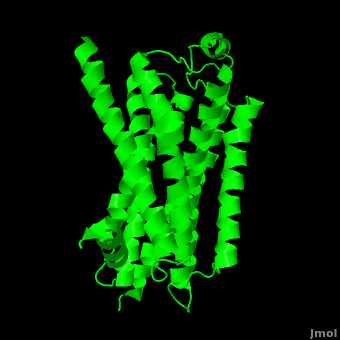
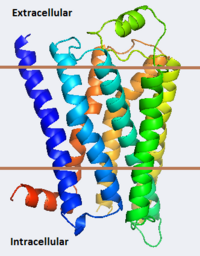
| - | As a family, GPCRs are renowned for their structure solution difficulty. In this model, Beta2-Adrenergic Receptor (B2AR) – T4 Lysozyme fusion was developed to allow for structure solution. Fortunately, the <scene name='Beta-2_Adrenergic_Receptor/Opening_lyso/1'>lysozyme portion</scene> of the structure does not appear to impact the structure of the B2AR. The structure of B2AR is very similar to the GPCR [[Rhodopsin]], which is a photoreceptor in the retina which allows the perception of light. B2AR is a transmembrane protein with <scene name='Beta-2_Adrenergic_Receptor/Helices/1'>7 transmembrane helices</scene> and an 8th helix which runs parallel to the cytoplasmic face of the membrane.<ref name="Rasmussen">PMID: 17952055</ref> Helices 2,5,6 and 7 of B2AR have <scene name='Beta-2_Adrenergic_Receptor/Kinks/1'>kinks caused by prolines</scene> at conserved places, which are important for activation of G protein effectors. The <scene name='Beta-2_Adrenergic_Receptor/Extracellular_residues/1'>extracellular regions</scene> on all GPCRs dictate the ligand specificity of GPCRs. The partial inverse agonist, Carazolol, <scene name='Beta-2_Adrenergic_Receptor/Cara_binding/1'>binds to the receptor binding pocket</scene> of B2AR, reducing the basal activity of the receptor. This interaction involves residues Tyr 199, Ser 203, Ser 207, Phe 193, Ser 204, Ser 293, Phe 290, Tyr 308, Phe 289, Asn 312, Tyr 316, Trp 286, Trp 109 and Asp 113. Carazolol occupies a similar position as the rhodopsin inverse agonist, retinal. Carazolol does not act with the so called “toggle switch” on helix 6, but does interact with Phe 290, causing <scene name='Beta-2_Adrenergic_Receptor/Cara_binding_w/1'>Trp 286 to assume an inactive rotameric state</scene>, effectively inhibiting B2AR activity.<ref>PMID: 17962520</ref> A | + | As a family, GPCRs are renowned for their structure solution difficulty. In this model, Beta2-Adrenergic Receptor (B2AR) – T4 Lysozyme fusion was developed to allow for structure solution. Fortunately, the <scene name='Beta-2_Adrenergic_Receptor/Opening_lyso/1'>lysozyme portion</scene> of the structure does not appear to impact the structure of the B2AR. The structure of B2AR is very similar to the GPCR [[Rhodopsin]], which is a photoreceptor in the retina which allows the perception of light. B2AR is a transmembrane protein with <scene name='Beta-2_Adrenergic_Receptor/Helices/1'>7 transmembrane helices</scene> and an 8th helix which runs parallel to the cytoplasmic face of the membrane.<ref name="Rasmussen">PMID: 17952055</ref> Helices 2,5,6 and 7 of B2AR have <scene name='Beta-2_Adrenergic_Receptor/Kinks/1'>kinks caused by prolines</scene> at conserved places, which are important for activation of G protein effectors. The <scene name='Beta-2_Adrenergic_Receptor/Extracellular_residues/1'>extracellular regions</scene> on all GPCRs dictate the ligand specificity of GPCRs. The partial inverse agonist, Carazolol, <scene name='Beta-2_Adrenergic_Receptor/Cara_binding/1'>binds to the receptor binding pocket</scene> of B2AR, reducing the basal activity of the receptor. This interaction involves residues Tyr 199, Ser 203, Ser 207, Phe 193, Ser 204, Ser 293, Phe 290, Tyr 308, Phe 289, Asn 312, Tyr 316, Trp 286, Trp 109 and Asp 113. Carazolol occupies a similar position as the rhodopsin inverse agonist, retinal. Carazolol does not act with the so called “toggle switch” on helix 6, but does interact with Phe 290, causing <scene name='Beta-2_Adrenergic_Receptor/Cara_binding_w/1'>Trp 286 to assume an inactive rotameric state</scene>, effectively inhibiting B2AR activity.<ref>PMID: 17962520</ref> A <scene name='Beta-2_Adrenergic_Receptor/Dry/1'>conserved DRY motif</scene> (residues 130-132) is present in all GPCRs.<ref>PMID: 18547522</ref> In rhodopsin, the Arginine in this motif forms a salt bridge with a glutamate, an interaction that maintains rhodopsin in its inactive state until it is exposed to light. In B2AR, Arg 131 <scene name='Beta-2_Adrenergic_Receptor/Dry_no_salt/1'>does not interact with Glu 268</scene>, which helps explain why B2AR has basal activity. Interestingly, Rhodopsin has no basil activity, a feature that is critical for vision.<ref>PMID: 18818650</ref> |
====Pharmaceutical Implications==== | ====Pharmaceutical Implications==== | ||
Revision as of 00:11, 4 April 2011
| |||||||||||
References
- ↑ 1.0 1.1 1.2 Witter FR, Zimmerman AW, Reichmann JP, Connors SL. In utero beta 2 adrenergic agonist exposure and adverse neurophysiologic and behavioral outcomes. Am J Obstet Gynecol. 2009 Dec;201(6):553-9. PMID:19961985 doi:10.1016/j.ajog.2009.07.010
- ↑ 2.0 2.1 Mehler MF, Purpura DP. Autism, fever, epigenetics and the locus coeruleus. Brain Res Rev. 2009 Mar;59(2):388-92. Epub 2008 Nov 24. PMID:19059284 doi:10.1016/j.brainresrev.2008.11.001
- ↑ Rasmussen SG, Choi HJ, Rosenbaum DM, Kobilka TS, Thian FS, Edwards PC, Burghammer M, Ratnala VR, Sanishvili R, Fischetti RF, Schertler GF, Weis WI, Kobilka BK. Crystal structure of the human beta2 adrenergic G-protein-coupled receptor. Nature. 2007 Nov 15;450(7168):383-7. Epub 2007 Oct 21. PMID:17952055 doi:10.1038/nature06325
- ↑ Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Kuhn P, Weis WI, Kobilka BK, Stevens RC. High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science. 2007 Nov 23;318(5854):1258-65. Epub 2007 Oct 25. PMID:17962520
- ↑ Hanson MA, Cherezov V, Griffith MT, Roth CB, Jaakola VP, Chien EY, Velasquez J, Kuhn P, Stevens RC. A specific cholesterol binding site is established by the 2.8 A structure of the human beta2-adrenergic receptor. Structure. 2008 Jun;16(6):897-905. PMID:18547522 doi:10.1016/j.str.2008.05.001
- ↑ Scheerer P, Park JH, Hildebrand PW, Kim YJ, Krauss N, Choe HW, Hofmann KP, Ernst OP. Crystal structure of opsin in its G-protein-interacting conformation. Nature. 2008 Sep 25;455(7212):497-502. PMID:18818650 doi:10.1038/nature07330
- ↑ Warne T, Moukhametzianov R, Baker JG, Nehme R, Edwards PC, Leslie AG, Schertler GF, Tate CG. The structural basis for agonist and partial agonist action on a beta(1)-adrenergic receptor. Nature. 2011 Jan 13;469(7329):241-4. PMID:21228877 doi:10.1038/nature09746
- ↑ Cruickshank JM. Beta blockers in hypertension. Lancet. 2010 Aug 7;376(9739):415; author reply 415-6. PMID:20692524 doi:10.1016/S0140-6736(10)61217-2
Proteopedia Page Contributors and Editors (what is this?)
Wayne Decatur, David Canner, Alexander Berchansky, Dotan Shaniv, Joel L. Sussman, Michal Harel