This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
User:Gregory Hoeprich/Sandbox 1
From Proteopedia
| Line 1: | Line 1: | ||
<references/><references/>{{STRUCTURE_1c1g|PDB=1c1g|SCENE=}} | <references/><references/>{{STRUCTURE_1c1g|PDB=1c1g|SCENE=}} | ||
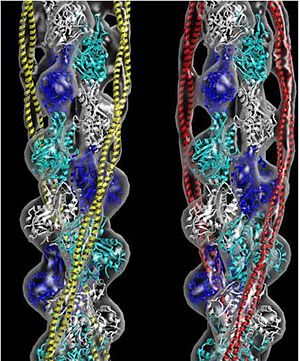
| - | [[Image:Tropomyosin Dimer-2.png | thumb | left| 300x180px | alt text | '''Tropomyosin:''' Coiled-Coil Dimer, which is composed of two alpha helices [http://www.pdb.org/pdb/explore/explore.do?structureId=1C1G (1C1G)] ]][[Image:B Lehman1.jpg | thumb | 300 x 430px | left | alt text | '''Tropomyosin''' (seen in yello and red) wrapped around actin filaments, which are EM reconstructions with G-actin ribbion structures filling in the EM structure). (Picture generated from William Lehman's [http://www.bumc.bu.edu/phys-biophys/people/faculty/lehman/ Website]) ]]'''[[Tropomyosin]] (TM)''' is an [[actin]] binding protein, which consists of a coiled-coil dimer (see left) and forms a polymer along the length of actin by a head-to-tail overlap (along the major grove of actin, see down & left)<ref>Tropomyosins. I. Gunning, Peter, 1950- II. Series.[DNLM: 1. Tropomyosin. W1 AD559 v.644 2008 / WE 500 T856 2008]</ref>. The head-to-tail overlap allows flexibility between the tropomyosin dimers so it will lay unstrained along the filament<ref name="Gunning"/>. Each tropomyosin molecule spans seven actin monomers within a filament and lays N- to C- terminally from actin's pointed to barbed end<ref name="Frye">PMID:20465283<ref/>. The 284 amino acid helix has a length of 420 Angstroms and has a molecular weight around 65-70 kilodaltons (vertebrate tropomyosin)<ref name="Gunning"/><ref name="Whitby">PMID:10651038<ref/>. A few of tropomyosin's characteristics as an actin binding protein includes regulation, stabilization and recruitment. | + | [[Image:Tropomyosin Dimer-2.png | thumb | left| 300x180px | alt text | '''Tropomyosin:''' Coiled-Coil Dimer, which is composed of two alpha helices [http://www.pdb.org/pdb/explore/explore.do?structureId=1C1G (1C1G)] ]][[Image:B Lehman1.jpg | thumb | 300 x 430px | left | alt text | '''Tropomyosin''' (seen in yello and red) wrapped around actin filaments, which are EM reconstructions with G-actin ribbion structures filling in the EM structure). (Picture generated from William Lehman's [http://www.bumc.bu.edu/phys-biophys/people/faculty/lehman/ Website]) ]]'''[[Tropomyosin]] (TM)''' is an [[actin]] binding protein, which consists of a coiled-coil dimer (see left) and forms a polymer along the length of actin by a head-to-tail overlap (along the major grove of actin, see down & left)<ref name="Gunning">Tropomyosins. I. Gunning, Peter, 1950- II. Series.[DNLM: 1. Tropomyosin. W1 AD559 v.644 2008 / WE 500 T856 2008]</ref>. The head-to-tail overlap allows flexibility between the tropomyosin dimers so it will lay unstrained along the filament<ref name="Gunning"/>. Each tropomyosin molecule spans seven actin monomers within a filament and lays N- to C- terminally from actin's pointed to barbed end<ref name="Frye">PMID:20465283<ref/>. The 284 amino acid helix has a length of 420 Angstroms and has a molecular weight around 65-70 kilodaltons (vertebrate tropomyosin)<ref name="Gunning"/><ref name="Whitby">PMID:10651038<ref/>. A few of tropomyosin's characteristics as an actin binding protein includes regulation, stabilization and recruitment. |
<br /> | <br /> | ||
Its role in muscle mechanics has been well established as it is a major regulatory component of the contractile apparatus, but its role in non-muscle systems is becoming evermore clear. In mammals, there are at least 40 known isoforms, which are generated by alternative splicing of multiple genes. These isoforms in non-muscle systems contribute to actin's stability by increasing filament rigidity and protecting the filament from actin severing proteins, like [[gelsolin]] or [http://en.wikipedia.org/wiki/Cofilin cofilin]. The different isoforms also aid in recruitment of various proteins, including myosin (a family of molecular motors). The reason for tropomyosin's diversity is not well known, but it is thought to exist to function at different developmental stages in some species as well as function in specific cells of higher multicellular organisms. | Its role in muscle mechanics has been well established as it is a major regulatory component of the contractile apparatus, but its role in non-muscle systems is becoming evermore clear. In mammals, there are at least 40 known isoforms, which are generated by alternative splicing of multiple genes. These isoforms in non-muscle systems contribute to actin's stability by increasing filament rigidity and protecting the filament from actin severing proteins, like [[gelsolin]] or [http://en.wikipedia.org/wiki/Cofilin cofilin]. The different isoforms also aid in recruitment of various proteins, including myosin (a family of molecular motors). The reason for tropomyosin's diversity is not well known, but it is thought to exist to function at different developmental stages in some species as well as function in specific cells of higher multicellular organisms. | ||
Revision as of 23:33, 9 May 2011
| |||||||||
| 1c1g, resolution 7.00Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||

Evolutionary Conservation
Solved Tropomyosin Structures
3mtu, 3mud – cTPM alpha-1 – chicken
1ic2 - cTPM alpha-1 (mutant)
2w49, 2w4u – cTnnC+cTnnT+cTnnI+cTPM alpha-1+cActin
2z5h – yTPM alpha-1 N-terminal+C-terminal+GNC4 leucine zipper+TnnT – yeast
2z5i - yTPM alpha-1 N-terminal+C-terminal+GNC4 leucine zipper
2efr, 2efs, 2d3e - rTPM alpha-1 C-terminal+GNC4 leucine zipper – rabbit
1kql - TPM alpha-1 C-terminal+GNC4 leucine zipper - rat
1mv4 - TPM alpha-1 C-terminal – rat
2g9j - TPM alpha-1 TM9A+GNC4 – rat
2b9c – TPM mid region – rat
1c1g – TPM – pig
2tma – TPM - model
References
- ↑ 1.0 1.1 Tropomyosins. I. Gunning, Peter, 1950- II. Series.[DNLM: 1. Tropomyosin. W1 AD559 v.644 2008 / WE 500 T856 2008]
- ↑ PMID:20465283<ref></ref>. The 284 amino acid helix has a length of 420 Angstroms and has a molecular weight around 65-70 kilodaltons (vertebrate tropomyosin)<ref></ref><ref>PMID:10651038<ref/>. A few of tropomyosin's characteristics as an actin binding protein includes regulation, stabilization and recruitment.
<br />
Its role in muscle mechanics has been well established as it is a major regulatory component of the contractile apparatus, but its role in non-muscle systems is becoming evermore clear. In mammals, there are at least 40 known isoforms, which are generated by alternative splicing of multiple genes. These isoforms in non-muscle systems contribute to actin's stability by increasing filament rigidity and protecting the filament from actin severing proteins, like [[gelsolin]] or [http://en.wikipedia.org/wiki/Cofilin cofilin]. The different isoforms also aid in recruitment of various proteins, including myosin (a family of molecular motors). The reason for tropomyosin's diversity is not well known, but it is thought to exist to function at different developmental stages in some species as well as function in specific cells of higher multicellular organisms.
<br />
<br />
The details of its structure/function relationship defines its role in different cell systems and disease states, this page further discuss tropomyosin's structure and function.
== Structure/Function Relationships ==
[[Image:Tropomyosin Alpha helix.png | thumb | right | 250x380px | alt text | '''Tropomyosin's''' Alpha Helices are represented in ribbon (Chain A) and ball and stick form (Chain B, without side chains). The yellow lines represent the polar contacts within the protein backbone, which stabilize the helical structure.]]As determined by the '''S'''tructural '''C'''lassification '''o'''f '''P'''rotein's ([http://scop.mrc-lmb.cam.ac.uk/scop/ '''SCOP''']) Database, Tropomyosin is categorized as follows (general to specific):
- '''Class:''' coiled-coil
- '''Fold:''' parallel coiled-coil
- '''Superfamily:''' tropomyosin
- '''Family:''' pig [[http://www.pdb.org/pdb/explore/explore.do?structureId=1C1G 1c1g]]

