User:Karen Lee/Sandbox 1
From Proteopedia
m |
(Editing that paragraph on sRB and nRB) |
||
| Line 18: | Line 18: | ||
===Key Features of RTP=== | ===Key Features of RTP=== | ||
| + | The function RTP-DNA complex requires two dimers of RTP. Dimerisation occurs through the extended α4-helix of each monomer forming an antiparallel coiled coil. It is thought that upon binding to the ''Ter'' sequence, the complex is able to impede the progress of replicative machinery headed by the replicative helicase. This is supported by the fact that RTP interacts with DnaB helicase from in ''E. coli'' ''in vitro'' and suggests an interaction with the ''B. subtilis'' equivalent<ref>Gautam, A., Mulugu, S., Alexander, K. & Bastia, D. (2001) A Single Domain of the Replication Termination Protein of ''Bacillus subtilis'' Is Involved in Arresting Both DnaB Helicase and RNA Polymerase. ''J. Biol. Chem.'' '''276(26)''', 23471-23479.</ref>. | ||
| - | + | Initial reports of the RTP-DNA structure were performed on a ''TerI'' (the first ''Ter'' site that the clockwise replication fork encounters) B site (sRB) which was symmetric in sequence<ref name="Wilce">Wilce, J. A., Vivian, J. P., Hastings, A. F., Otting, G., Folmer, R. H., Duggin, I. G. ''et al.'' (2001) Structure of the RTP-DNA complex and the mechanism of polar replication fork arrest. ''Nature Struct. Biol.'' '''8''', 206-210.</ref>. NMR and crystallography revealed a symmetric RTP bound to the symmetric DNA site. However, further studies on the native ''TerI'' B site (nRB) showed an asymmetric structure. 6 base pair differences between the sRB and nRB define the native site: three upstream and three downstream, leading to an asymmetric RTP binding to the site. What is of importance is that the RTP-sRB interaction showed similar binding affinity to the RTP-nRB interaction, but had reduced fork arrest capability<ref name="Wilce" />. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| + | This differential capability is due to the location of the 6 base pair changes. The three downstream changes have no bearing on the conformation of RTP as no base-specific interactions are made in this region. However, the three upstream changes are located within the major groove of the dsDNA of the ''TerI'' site. This is where the α3 helix binds which underlies RTP binding specificity<ref name="Vivian" />. This affects the binding of the RTP monomer to the upstream region and initiates the structural difference observed. | ||
| + | This leads into the fact that RTP binds asymmetrically across the nRB site and therefore allows the complex to act as a polar barrier to the replication fork. When the fork approaches the B site (with tight RTP-DNA binding) the fork is unable to progress and is paused. Approaching from the A site does not impede its progress. This polarity can be explained by both the differential binding affinity as explained above and the cooperative binding affect of the RTP monomers. The complex formed between an RTP dimer and the B site facilitates cooperative binding of another RTP dimer to the A site to form a complete RTP-Ter complex<ref name="Vivian" />. | ||
==Tus== | ==Tus== | ||
| Line 31: | Line 30: | ||
===Structure of Tus=== | ===Structure of Tus=== | ||
| - | The Tus protein works as a monomer, each monomer consists of two domains, the amino and carboxy domains. The overall structure of Tus is made up of two <scene name='User:Karen_Lee/Sandbox_1/Tus_withdna_alphahelices/1'>two α-helical regions</scene> separated by central twisted anti-parallel <scene name='User:Karen_Lee/Sandbox_1/Tus_withdna_betasheets/1'>β-strands</scene>. These structures together form a <scene name='User:Karen_Lee/Sandbox_1/Tus_nodna/1'>positively charged central cleft</scene> that accommodates a B-form Ter <scene name='User:Karen_Lee/Sandbox_1/Tus_withdna/1'>DNA duplex</scene>. This binding involves two β-strands involes contacting the bases and sugar-phosphate back bones from the major groove of the Ter DNA. Upon Tus binding, the major groove becomes deeper and the minor groove is significantly deeper, causing the DNA to be slightly bent. | + | The Tus protein works as a monomer, each monomer consists of two domains, the amino and carboxy domains. The overall structure of Tus is made up of two <scene name='User:Karen_Lee/Sandbox_1/Tus_withdna_alphahelices/1'>two α-helical regions</scene> separated by central twisted anti-parallel <scene name='User:Karen_Lee/Sandbox_1/Tus_withdna_betasheets/1'>β-strands</scene>. These structures together form a <scene name='User:Karen_Lee/Sandbox_1/Tus_nodna/1'>positively charged central cleft</scene> that accommodates a B-form ''Ter'' <scene name='User:Karen_Lee/Sandbox_1/Tus_withdna/1'>DNA duplex</scene>. This binding involves two β-strands involes contacting the bases and sugar-phosphate back bones from the major groove of the ''Ter'' DNA. Upon Tus binding, the major groove becomes deeper and the minor groove is significantly deeper, causing the DNA to be slightly bent. |
| Line 58: | Line 57: | ||
| - | =References= | + | ==References== |
<references /> | <references /> | ||
Revision as of 10:37, 14 May 2011
Contents |
Replication Termination
Replication is an essential process in all cells. The process copies the chromosomal DNA of the organism to provide the extra copy needed in cell division and is therefore critical in the biological inheritance of genes. In cells with circular chromosomes, replication starts from a single origin proceed with two replication forks moving in opposite directions. It should follow that this process must be terminated or multiple copies of the chromosome would be made.
The process of terminating replication is performed by replication termination proteins. These proteins bind to specific sequences in the DNA, called Ter sites. This binding provides a physical blockage in the DNA that stops the replication machinery. In B. subtilis, the termination protein is called Replication Terminator Protein (RTP), and in E. coli it is Termination Utilisation Substance (Tus).
In each circular chromosome, there are two sets of Ter sites that appear roughly opposite to the origin of replication. One set blocks the clockwise replication fork while the other traps the anti-clockwise replication fork. Interestingly, both these proteins bind to DNA in such a way that they terminate the replication fork in one direction, but simultaneously allow the replication fork to continue in the other direction.
RTP
|
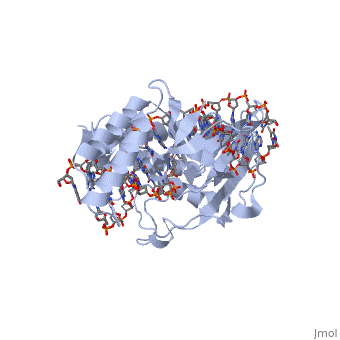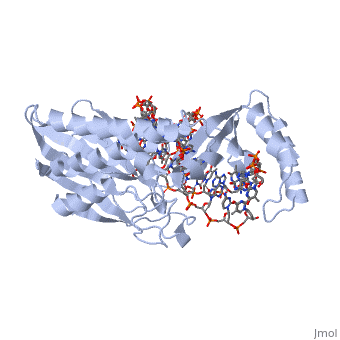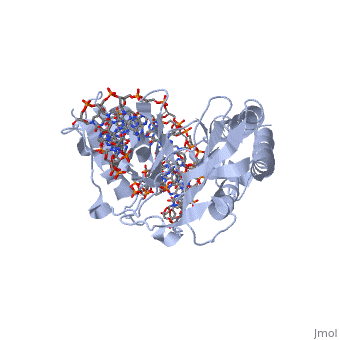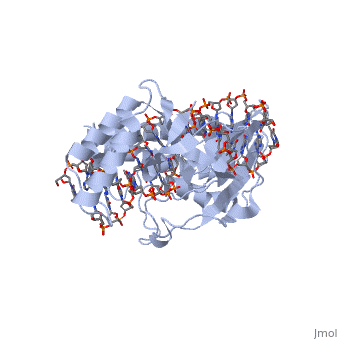
Structure of RTP
A single RTP monomer consists of four , three and an unstructured domain. The binds to DNA by into the major groove of Ter sites, while the interacts with the minor groove. The N-terminal arm also binds to the Ter site[1].
RTP conforms to the classic winged-helix motif, a compact α/β structure with αβααββ topology where "" project from the loop between β2 and β3-strands[2]. However, each monomer lacks a well-formed β1-strand (named β1-loop) and has an additional α4-helix following the β3-strand[3].
(.)
Key Features of RTP
The function RTP-DNA complex requires two dimers of RTP. Dimerisation occurs through the extended α4-helix of each monomer forming an antiparallel coiled coil. It is thought that upon binding to the Ter sequence, the complex is able to impede the progress of replicative machinery headed by the replicative helicase. This is supported by the fact that RTP interacts with DnaB helicase from in E. coli in vitro and suggests an interaction with the B. subtilis equivalent[4].
Initial reports of the RTP-DNA structure were performed on a TerI (the first Ter site that the clockwise replication fork encounters) B site (sRB) which was symmetric in sequence[5]. NMR and crystallography revealed a symmetric RTP bound to the symmetric DNA site. However, further studies on the native TerI B site (nRB) showed an asymmetric structure. 6 base pair differences between the sRB and nRB define the native site: three upstream and three downstream, leading to an asymmetric RTP binding to the site. What is of importance is that the RTP-sRB interaction showed similar binding affinity to the RTP-nRB interaction, but had reduced fork arrest capability[5].
This differential capability is due to the location of the 6 base pair changes. The three downstream changes have no bearing on the conformation of RTP as no base-specific interactions are made in this region. However, the three upstream changes are located within the major groove of the dsDNA of the TerI site. This is where the α3 helix binds which underlies RTP binding specificity[3]. This affects the binding of the RTP monomer to the upstream region and initiates the structural difference observed.
This leads into the fact that RTP binds asymmetrically across the nRB site and therefore allows the complex to act as a polar barrier to the replication fork. When the fork approaches the B site (with tight RTP-DNA binding) the fork is unable to progress and is paused. Approaching from the A site does not impede its progress. This polarity can be explained by both the differential binding affinity as explained above and the cooperative binding affect of the RTP monomers. The complex formed between an RTP dimer and the B site facilitates cooperative binding of another RTP dimer to the A site to form a complete RTP-Ter complex[3].
Tus
|
Structure of Tus
The Tus protein works as a monomer, each monomer consists of two domains, the amino and carboxy domains. The overall structure of Tus is made up of two separated by central twisted anti-parallel . These structures together form a that accommodates a B-form Ter . This binding involves two β-strands involes contacting the bases and sugar-phosphate back bones from the major groove of the Ter DNA. Upon Tus binding, the major groove becomes deeper and the minor groove is significantly deeper, causing the DNA to be slightly bent.
References
- ↑ Pai, S. K., Bussiere, D. E., Wang, F., Hutchinson, C. A., White, S. W. & Bastia, D. (1996) The structure and function of the replication terminator protein of Bacillus subtilis: identification of the ‘winged helix’ DNA-binding domain. EMBO J. 15(12), 3164-3173.
- ↑ Gajiwala, K. S. & Burley, S. K. (2000) Winged Helix proteins. Curr. Opin. Struct. Biol. 10, 110-116.
- ↑ 3.0 3.1 3.2 Vivian, J. P., Porter, C. J., Wilce, J. A. & Wilce, M. C. J. (2007) An Asymmetric Structure of the Bacillus subtilis Replication Terminator Protein in Complex with DNA. J. Mol. Biol. 370, 481-491.
- ↑ Gautam, A., Mulugu, S., Alexander, K. & Bastia, D. (2001) A Single Domain of the Replication Termination Protein of Bacillus subtilis Is Involved in Arresting Both DnaB Helicase and RNA Polymerase. J. Biol. Chem. 276(26), 23471-23479.
- ↑ 5.0 5.1 Wilce, J. A., Vivian, J. P., Hastings, A. F., Otting, G., Folmer, R. H., Duggin, I. G. et al. (2001) Structure of the RTP-DNA complex and the mechanism of polar replication fork arrest. Nature Struct. Biol. 8, 206-210.