User:Karen Lee/Sandbox 1
From Proteopedia
| Line 24: | Line 24: | ||
All of the ''Ter'' sites in the ''B. subtilis'' chromosome are only partially symmetric about the mid-point. However, initial studies of the RTP-DNA structure were performed using a symmetrically designed sequence (sRB) based on the ''TerI'' (the first ''Ter'' site that the clockwise replication fork encounters) B site <ref name="Wilce">Wilce, J. A., Vivian, J. P., Hastings, A. F., Otting, G., Folmer, R. H., Duggin, I. G. ''et al.'' (2001) Structure of the RTP-DNA complex and the mechanism of polar replication fork arrest. ''Nature Struct. Biol.'' '''8''', 206-210.</ref>. NMR and crystallography revealed that RTP bound symmetrically to this symmetric sRB sequence. However, further studies using the non-symmetric native ''TerI'' B site (nRB) showed an asymmetric structure. Between the sRB and nRB sites there were only 6 base pair differences: three upstream and three downstream, but these changes were significant enough to influence how RTP binds to the DNA. Despite the change in sequence, the RTP-sRB interaction showed similar binding affinity to the RTP-nRB interaction, but had reduced fork arrest capability<ref name="Wilce" />. | All of the ''Ter'' sites in the ''B. subtilis'' chromosome are only partially symmetric about the mid-point. However, initial studies of the RTP-DNA structure were performed using a symmetrically designed sequence (sRB) based on the ''TerI'' (the first ''Ter'' site that the clockwise replication fork encounters) B site <ref name="Wilce">Wilce, J. A., Vivian, J. P., Hastings, A. F., Otting, G., Folmer, R. H., Duggin, I. G. ''et al.'' (2001) Structure of the RTP-DNA complex and the mechanism of polar replication fork arrest. ''Nature Struct. Biol.'' '''8''', 206-210.</ref>. NMR and crystallography revealed that RTP bound symmetrically to this symmetric sRB sequence. However, further studies using the non-symmetric native ''TerI'' B site (nRB) showed an asymmetric structure. Between the sRB and nRB sites there were only 6 base pair differences: three upstream and three downstream, but these changes were significant enough to influence how RTP binds to the DNA. Despite the change in sequence, the RTP-sRB interaction showed similar binding affinity to the RTP-nRB interaction, but had reduced fork arrest capability<ref name="Wilce" />. | ||
| - | This | + | This differential capability fork arrest is due to the location of the 6 base pair changes. The three downstream changes have no bearing on the conformation of RTP as no base-specific interactions are made in this region. However, the three upstream changes are located within the major groove of the dsDNA of the ''TerI'' site, where the <scene name='User:Karen_Lee/Sandbox_1/Rtp_alpha_helix_3_dna/2'>α3 helix</scene> binds. Thus, it is these 3 bases that underlies the RTP binding specificity<ref name="Vivian" />. Changing these 3 crucial bases affected the binding of the RTP monomer to the upstream region and initiated the observed structural difference. |
As RTP binds asymmetrically across the nRB, due to its differing binding affinities for the B and A sites, a polar complex is formed to block the replication fork. When the fork approaches the B site where there is tight RTP-DNA binding, the fork is unable to progress. But approaching from the A site, where there is a lower binding affinity, the fork is able to pass the complex. | As RTP binds asymmetrically across the nRB, due to its differing binding affinities for the B and A sites, a polar complex is formed to block the replication fork. When the fork approaches the B site where there is tight RTP-DNA binding, the fork is unable to progress. But approaching from the A site, where there is a lower binding affinity, the fork is able to pass the complex. | ||
===RTP as a checkpoint regulator=== | ===RTP as a checkpoint regulator=== | ||
| - | An interesting feature is that RTP can cause replication fork pausing in ''B. subtilis'' at regions other than ''Ter'' sites. There appear to be stringent terminator (''STer'') regions where RTP can bind and arrest replication. These regions are located around 200 kb either side of the origin of replication<ref>Levine, A., Vannier, F., Dehbi, M., Henckes, G. & Seror, S. J. (1991) The stringent response blocks DNA replication outside the ''ori'' region in ''Bacillus subtilis'' and at the origin in ''Escherichia coli''. ''J. Mol. Biol.'' '''219''', 605-613.</ref>. Studies have shown that there is 76% homology between this site and the | + | An interesting feature is that RTP can cause replication fork pausing in ''B. subtilis'' at regions other than ''Ter'' sites, acting as a replication checkpoint regulator. There appear to be stringent terminator (''STer'') regions where RTP can bind and arrest replication when something is wrong. These regions are located around 200 kb either side of the origin of replication<ref>Levine, A., Vannier, F., Dehbi, M., Henckes, G. & Seror, S. J. (1991) The stringent response blocks DNA replication outside the ''ori'' region in ''Bacillus subtilis'' and at the origin in ''Escherichia coli''. ''J. Mol. Biol.'' '''219''', 605-613.</ref>. Studies have shown that there is 76% homology between this site and the regular ''Ter'' sites<ref>Autret, S., Levine, A., Vannier, F., Fujita, Y. & Seror, S. J. (1999) The replication checkpoint control in ''Bacillus subtilis'': identification of a novel RTP-binding sequence essential for the replication fork arrest after induction of the stringent response. ''Mol. Microbiol.'', '''31''', 1665-1679.</ref>. Additionally, there is no need for two dimers to interact ''in vitro'', which avoids unwanted arrest during normal replication. |
==Tus== | ==Tus== | ||
| Line 35: | Line 35: | ||
===Structure of Tus=== | ===Structure of Tus=== | ||
| - | The Tus protein works as a monomer, each monomer consists of two domains, the amino and carboxy domains. The overall structure of Tus is made up of two <scene name='User:Karen_Lee/Sandbox_1/Tus_withdna_alphahelices/1'>two α-helical regions</scene> separated by central twisted anti-parallel <scene name='User:Karen_Lee/Sandbox_1/Tus_withdna_betasheets/1'>β-strands</scene>. These structures together form a <scene name='User:Karen_Lee/Sandbox_1/Tus_nodna/1'>positively charged central cleft</scene> that accommodates a B-form ''Ter'' <scene name='User:Karen_Lee/Sandbox_1/Tus_withdna/1'>DNA duplex</scene>. This binding involves two β-strands | + | The Tus protein works as a monomer, each monomer consists of two domains, the amino and carboxy domains. The overall structure of Tus is made up of two <scene name='User:Karen_Lee/Sandbox_1/Tus_withdna_alphahelices/1'>two α-helical regions</scene> separated by central twisted anti-parallel <scene name='User:Karen_Lee/Sandbox_1/Tus_withdna_betasheets/1'>β-strands</scene>. These structures together form a <scene name='User:Karen_Lee/Sandbox_1/Tus_nodna/1'>positively charged central cleft</scene> that accommodates a B-form ''Ter'' <scene name='User:Karen_Lee/Sandbox_1/Tus_withdna/1'>DNA duplex</scene>. This binding involves two β-strands contacting the bases and sugar-phosphate back bones from the major groove of the ''Ter'' DNA. Upon Tus binding, the major groove becomes deeper and the minor groove is significantly deeper, causing the DNA to be slightly bent. |
Revision as of 07:07, 15 May 2011
Contents |
Replication Termination
Replication is an essential process in all cells. The process copies the chromosomal DNA of the organism to provide the extra copy needed in cell division and is therefore critical in the biological inheritance of genes. In cells with circular chromosomes, replication starts from a single origin proceed with two replication forks moving in opposite directions. It should follow that this process must be terminated or multiple copies of the chromosome would be made.
The process of terminating replication is performed by replication termination proteins. These proteins bind to specific sequences in the DNA, called Ter sites. This binding provides a physical blockage in the DNA that stops the replication machinery. In B. subtilis, the termination protein is called Replication Terminator Protein (RTP), and in E. coli it is Termination Utilisation Substance (Tus).
In each circular chromosome, there are two sets of Ter sites that appear roughly opposite to the origin of replication. One set blocks the clockwise replication fork while the other traps the anti-clockwise replication fork. Interestingly, both these proteins bind to DNA in such a way that they terminate the replication fork in one direction, but simultaneously allow the replication fork to continue in the other direction.
RTP
|
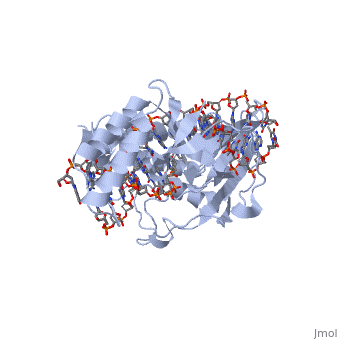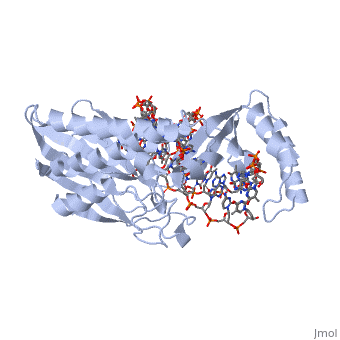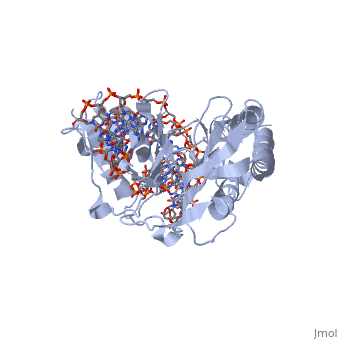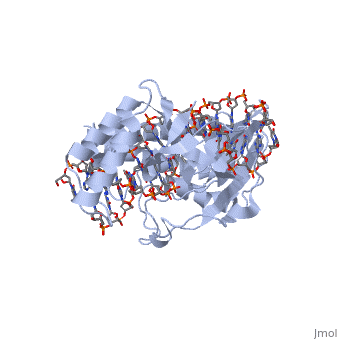
Structure of RTP
A single RTP monomer consists of four , three and an unstructured domain. The binds to DNA by into the major groove of Ter sites, while the interacts with the minor groove. The N-terminal arm also binds to the Ter site[1].
RTP conforms to the classic winged-helix motif, a compact α/β structure with αβααββ topology where "" project from the loop between and [2]. However, each monomer lacks a well-formed β1-strand (named β1-loop) and has an additional following the β3-strand[3].
(.)
Key Features of RTP
The functional RTP-DNA complex requires two dimers of RTP. Dimerisation occurs through the extended of each monomer forming an anti-parallel coiled coil. The RTP dimer binds to the B site first which then facilitates cooperative binding of another RTP dimer to the A site to form a complete RTP-Ter complex[3].
It is thought that upon binding to the Ter sequence, the complex is able to impede the progress of replicative machinery headed by the replicative helicase. This is supported by in vitro studies involving E. coli where RTP was shown to interact with the DnaB helicase, thus a similar interaction may also occur in B. subtilis [4].
All of the Ter sites in the B. subtilis chromosome are only partially symmetric about the mid-point. However, initial studies of the RTP-DNA structure were performed using a symmetrically designed sequence (sRB) based on the TerI (the first Ter site that the clockwise replication fork encounters) B site [5]. NMR and crystallography revealed that RTP bound symmetrically to this symmetric sRB sequence. However, further studies using the non-symmetric native TerI B site (nRB) showed an asymmetric structure. Between the sRB and nRB sites there were only 6 base pair differences: three upstream and three downstream, but these changes were significant enough to influence how RTP binds to the DNA. Despite the change in sequence, the RTP-sRB interaction showed similar binding affinity to the RTP-nRB interaction, but had reduced fork arrest capability[5].
This differential capability fork arrest is due to the location of the 6 base pair changes. The three downstream changes have no bearing on the conformation of RTP as no base-specific interactions are made in this region. However, the three upstream changes are located within the major groove of the dsDNA of the TerI site, where the binds. Thus, it is these 3 bases that underlies the RTP binding specificity[3]. Changing these 3 crucial bases affected the binding of the RTP monomer to the upstream region and initiated the observed structural difference.
As RTP binds asymmetrically across the nRB, due to its differing binding affinities for the B and A sites, a polar complex is formed to block the replication fork. When the fork approaches the B site where there is tight RTP-DNA binding, the fork is unable to progress. But approaching from the A site, where there is a lower binding affinity, the fork is able to pass the complex.
RTP as a checkpoint regulator
An interesting feature is that RTP can cause replication fork pausing in B. subtilis at regions other than Ter sites, acting as a replication checkpoint regulator. There appear to be stringent terminator (STer) regions where RTP can bind and arrest replication when something is wrong. These regions are located around 200 kb either side of the origin of replication[6]. Studies have shown that there is 76% homology between this site and the regular Ter sites[7]. Additionally, there is no need for two dimers to interact in vitro, which avoids unwanted arrest during normal replication.
Tus
|
Structure of Tus
The Tus protein works as a monomer, each monomer consists of two domains, the amino and carboxy domains. The overall structure of Tus is made up of two separated by central twisted anti-parallel . These structures together form a that accommodates a B-form Ter . This binding involves two β-strands contacting the bases and sugar-phosphate back bones from the major groove of the Ter DNA. Upon Tus binding, the major groove becomes deeper and the minor groove is significantly deeper, causing the DNA to be slightly bent.
References
- ↑ Pai, S. K., Bussiere, D. E., Wang, F., Hutchinson, C. A., White, S. W. & Bastia, D. (1996) The structure and function of the replication terminator protein of Bacillus subtilis: identification of the ‘winged helix’ DNA-binding domain. EMBO J. 15(12), 3164-3173.
- ↑ Gajiwala, K. S. & Burley, S. K. (2000) Winged Helix proteins. Curr. Opin. Struct. Biol. 10, 110-116.
- ↑ 3.0 3.1 3.2 Vivian, J. P., Porter, C. J., Wilce, J. A. & Wilce, M. C. J. (2007) An Asymmetric Structure of the Bacillus subtilis Replication Terminator Protein in Complex with DNA. J. Mol. Biol. 370, 481-491.
- ↑ Gautam, A., Mulugu, S., Alexander, K. & Bastia, D. (2001) A Single Domain of the Replication Termination Protein of Bacillus subtilis Is Involved in Arresting Both DnaB Helicase and RNA Polymerase. J. Biol. Chem. 276(26), 23471-23479.
- ↑ 5.0 5.1 Wilce, J. A., Vivian, J. P., Hastings, A. F., Otting, G., Folmer, R. H., Duggin, I. G. et al. (2001) Structure of the RTP-DNA complex and the mechanism of polar replication fork arrest. Nature Struct. Biol. 8, 206-210.
- ↑ Levine, A., Vannier, F., Dehbi, M., Henckes, G. & Seror, S. J. (1991) The stringent response blocks DNA replication outside the ori region in Bacillus subtilis and at the origin in Escherichia coli. J. Mol. Biol. 219, 605-613.
- ↑ Autret, S., Levine, A., Vannier, F., Fujita, Y. & Seror, S. J. (1999) The replication checkpoint control in Bacillus subtilis: identification of a novel RTP-binding sequence essential for the replication fork arrest after induction of the stringent response. Mol. Microbiol., 31, 1665-1679.