|
|
| Line 3: |
Line 3: |
| | <!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | | <!-- PLEASE ADD YOUR CONTENT BELOW HERE --> |
| | | | |
| - | =Trypsin=
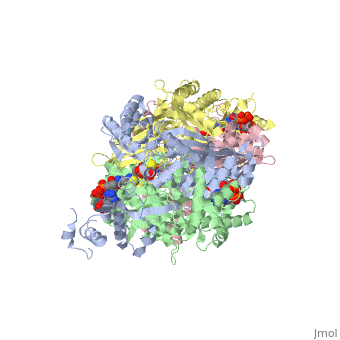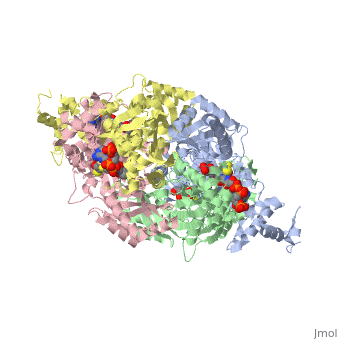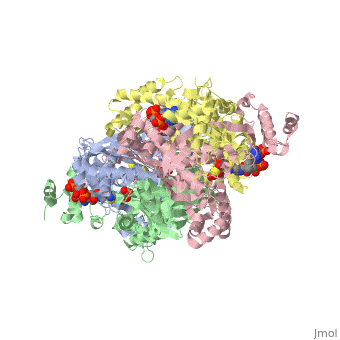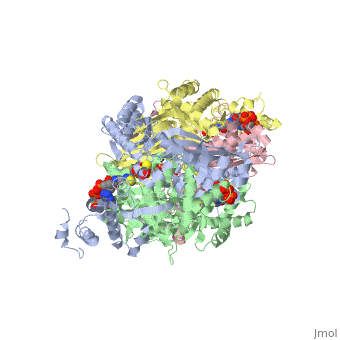
| + | Introduction<StructureSection load='1dq8' size='500' side='right' caption='Structure of HMG-CoA reductase (PDB entry [[9pap]])' scene=''><scene name='Sandbox_31/Hydrophobic_residues/1'>Hydrophobic Residues</scene> |
| - | Molecular Weight: 23.3 kDa
| + | </StructureSection> |
| - | ==Structural Aspects==
| + | |
| - | <applet scene='Sandbox_31/Trypsin_rainbow/2' size='225' frame='true' align='right' caption='The protein trypsin with various structures highlighted' /> | + | |
| - | | + | |
| - | There are a few structural aspects that are very important to trypsin. In the <scene name='Sandbox_31/Trypsin_rainbow/2'>rainbow trypsin</scene> image to the right, one can follow the lineage of the protein easily by tracing the protein from the N-terminus, which is colored blue, to the C-terminus, which is colored green. Other important aspects of this protein include: alpha helices, beta sheets, and the <scene name='Sandbox_31/Backbone/1'>backbone</scene>.
| + | |
| - | | + | |
| - | The <scene name='Sandbox_31/Secondary_structures/2'>secondary structures</scene> are the alpha helices and beta sheets that form after the primary structure, or the sequence of amino acids. In this image, the alpha helices are pink and the beta sheets are yellow.
| + | |
| - | | + | |
| - | In this image, only the <scene name='Sandbox_31/Alpha_helices/1'>alpha helices</scene> are highlighted.
| + | |
| - | | + | |
| - | There are two <scene name='Sandbox_31/Beta_sheets/2'>beta sheets</scene> in this protein as well, exhibiting a beta sheet-beta hairpin turn-beta sheet. These beta sheets are anti-parallel to one another.
| + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | ==Polar vs Nonpolar Residues==
| + | |
| - | This image reveals which molecules that are <scene name='Sandbox_31/Polarities/1'>polar and non-polar</scene>. As the molecule gets moved around, one can see that the polar molecules (orange) tend to be closer to the outside while the non-polar molecules (aqua) tend to curl towards the inside.
| + | |
| - | | + | |
| - | This molecule can also be displayed in a stick or wire frame format and exhibits whether the amino acid is <scene name='Sandbox_31/Hydrophobicity/2'>hydrophobic or hydrophilic</scene>. The hydrophobic molecules are in blue and the hydrophilic molecules are in orange. Again, it is seen that the hydrophobic molecules tend to concentrate on the inside of the protein while the hydrophilic amino acids are on or near the outside of the protein.
| + | |
| - | | + | |
| - | | + | |
| - | ==Attractions of Structural Components==
| + | |
| - | | + | |
| - | | + | |
| - | ==Ligands and Intermolecular Forces==
| + | |
| - | | + | |
| - | | + | |
| - | ==Function==
| + | |
| - | Trypsin hydrolyzes proteins and peptides. Trypsin acts on lysine and arginine; it cleaves the peptieds on the C-terminal side of the lysine and arginine residues.
| + | |