Sandbox reserve 103
From Proteopedia
| Line 2: | Line 2: | ||
<applet load='3omv' size='[450,338]' frame='true' align='right' | <applet load='3omv' size='[450,338]' frame='true' align='right' | ||
caption='C-Raf (3omv)' scene=''User:Thuy V. Nguyen/Sandbox103/Loadedfrompdb/4'/> | caption='C-Raf (3omv)' scene=''User:Thuy V. Nguyen/Sandbox103/Loadedfrompdb/4'/> | ||
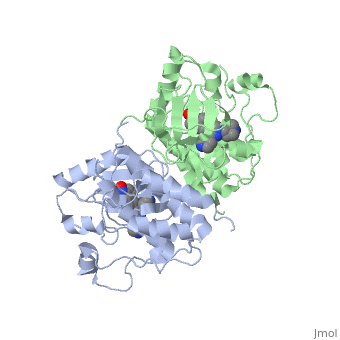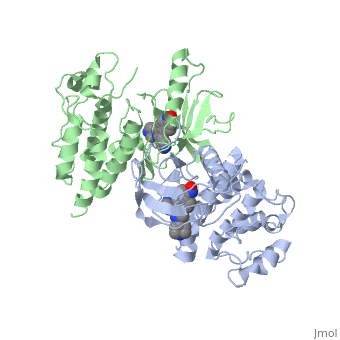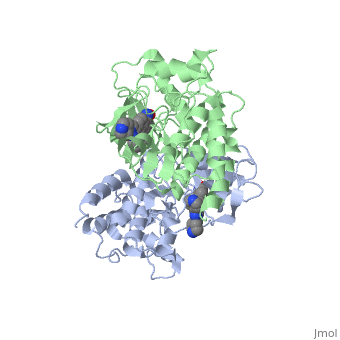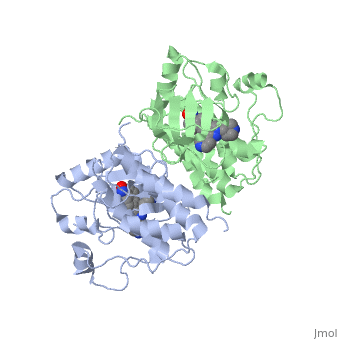
| - | '''C-Raf''' is | + | '''C-Raf''' (Raf1) is one of the isoforms of Raf serine/threonine kinase. The others are A-Raf and B-Raf. The Raf-kinase is activated by Ras protein. Activated Raf will trigger the phosphorylation of MEK and ERK, which regulates some transcription factors that induce gene expression required for cell proliferation. Raf1 activation is found in 50% of renal cell carcinoma and 100% of hepatocellular carcinoma (Scott Wilhelm et al., 2006). Sorafenib is an FDA approval small molecule for Raf1 inhibitor. |
| - | + | Insulin is made up of two pieces called the A- and B-chain, shown above in blue and green respectively. These two chains are joined by disulfide bonds, which are shown in yellow. This single piece made up of the A- and B-chains is the active form of the insulin hormone. This is the form that binds the insulin receptor on fat or muscle cells in the body, singling them to take up glucose, or sugar, from the blood and save it for later. | |
Insulin is able to pair-up with itself and form a dimer by forming hydrogen bonds between the ends of two B-chains. These <scene name='User:Whitney_Stoppel/sandbox1/Insulin_dimer/2'>hydrogen bonds</scene> are shown above in white. Then, 3 dimers can come together in the presence of zinc ions and form a hexamer. Insulin is stored in the <scene name='User:Whitney_Stoppel/sandbox1/Insulin_hexamer/4'>hexameric form</scene> in the body. This <scene name='User:Whitney_Stoppel/sandbox1/Insulin_ph7/2'>scene highlights</scene> the hydrophobic (gray) and polar (purple) parts of an insulin monomer at a pH of 7. It is believed that the hydrophobic sections on the B-chain cause insulin aggregation which initially caused problems in the manufacture and storage of insulin for [[Pharmaceutical_Drugs#Treatments|pharmaceutical use]]. | Insulin is able to pair-up with itself and form a dimer by forming hydrogen bonds between the ends of two B-chains. These <scene name='User:Whitney_Stoppel/sandbox1/Insulin_dimer/2'>hydrogen bonds</scene> are shown above in white. Then, 3 dimers can come together in the presence of zinc ions and form a hexamer. Insulin is stored in the <scene name='User:Whitney_Stoppel/sandbox1/Insulin_hexamer/4'>hexameric form</scene> in the body. This <scene name='User:Whitney_Stoppel/sandbox1/Insulin_ph7/2'>scene highlights</scene> the hydrophobic (gray) and polar (purple) parts of an insulin monomer at a pH of 7. It is believed that the hydrophobic sections on the B-chain cause insulin aggregation which initially caused problems in the manufacture and storage of insulin for [[Pharmaceutical_Drugs#Treatments|pharmaceutical use]]. | ||
</StructureSection> | </StructureSection> | ||
Revision as of 19:43, 16 December 2011
>One of the CBI Molecules being studied in the University of Massachusetts Amherst Chemistry-Biology Interface Program at UMass Amherst in the Peyton group and on display at the Molecular Playground.
|
C-Raf (Raf1) is one of the isoforms of Raf serine/threonine kinase. The others are A-Raf and B-Raf. The Raf-kinase is activated by Ras protein. Activated Raf will trigger the phosphorylation of MEK and ERK, which regulates some transcription factors that induce gene expression required for cell proliferation. Raf1 activation is found in 50% of renal cell carcinoma and 100% of hepatocellular carcinoma (Scott Wilhelm et al., 2006). Sorafenib is an FDA approval small molecule for Raf1 inhibitor.
Insulin is made up of two pieces called the A- and B-chain, shown above in blue and green respectively. These two chains are joined by disulfide bonds, which are shown in yellow. This single piece made up of the A- and B-chains is the active form of the insulin hormone. This is the form that binds the insulin receptor on fat or muscle cells in the body, singling them to take up glucose, or sugar, from the blood and save it for later.
Insulin is able to pair-up with itself and form a dimer by forming hydrogen bonds between the ends of two B-chains. These are shown above in white. Then, 3 dimers can come together in the presence of zinc ions and form a hexamer. Insulin is stored in the in the body. This the hydrophobic (gray) and polar (purple) parts of an insulin monomer at a pH of 7. It is believed that the hydrophobic sections on the B-chain cause insulin aggregation which initially caused problems in the manufacture and storage of insulin for pharmaceutical use. </StructureSection>