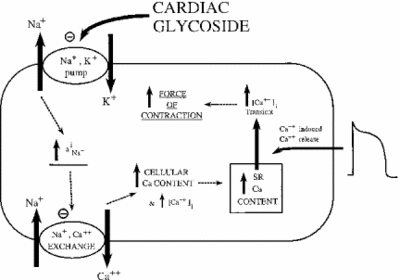
Ouabain
From Proteopedia
| Line 3: | Line 3: | ||
==Target Protein: Na+/K+ ATPase== | ==Target Protein: Na+/K+ ATPase== | ||
| - | [[Image:498px-Scheme sodium-potassium pump-en-2.svg.png| | + | [[Image:498px-Scheme sodium-potassium pump-en-2.svg.png|420px|right|upright=3.0]] |
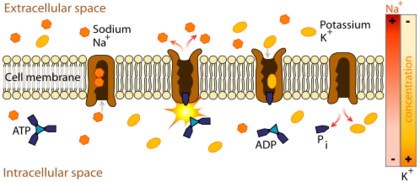
[[Na+/K+ ATPase]] actively transports potassium into the cell and sodium to the extra-cellular space according to the general scheme depicted below. This transmembrane protein is composed of three subunits, alpha, beta, and gamma, that may dimerize with other pumps ''in vivo''. The<scene name='Sandbox_60/Drug_in_complex_situated/1'>''in vitro''</scene>, crystal structure is a radially symmetrical hexamer, when in living tissues, the proteins most likely associate bilaterally. The alpha chain is shown in pink/blue and the beta chain in yellow/green. Of the two, the alpha subunit plays the leading role in the functioning of the protein. The bundle of alpha helices in the center of the protein position hydrophobic residues at the exterior of the motif, opening a portal through the lipid bilayer. It is inside this transmembrane domain that residues of the alpha subunit coordinate sodium or potassium ions. The large domain on the cytoplasmic side of the bilayer (furthest from beta subunit) floats in solution and is responsible for binding and hydrolyzing ATP. As with many protein enzymes of phosphoester hydrolysis, the phosphate groups are coordinated with a <scene name='Sandbox_60/Drug_in_complex_mg/1'>magnesium cofactor</scene>. The energy released from high-energy phosphate cleavage is transfered via conformational changes to the transmembrane domain, where ions are forced against their concentration gradient. On the extracellular side, the <scene name='Sandbox_60/Drug_in_complex_situated/1'>beta subunit</scene> moves to prevent the dissociation of potassium ions during their entry into the cell. A third gamma subunit (purple/orange), along with the beta subunit, contributes to the anchoring of transmembrane domains to the phospholipid bilayer. | [[Na+/K+ ATPase]] actively transports potassium into the cell and sodium to the extra-cellular space according to the general scheme depicted below. This transmembrane protein is composed of three subunits, alpha, beta, and gamma, that may dimerize with other pumps ''in vivo''. The<scene name='Sandbox_60/Drug_in_complex_situated/1'>''in vitro''</scene>, crystal structure is a radially symmetrical hexamer, when in living tissues, the proteins most likely associate bilaterally. The alpha chain is shown in pink/blue and the beta chain in yellow/green. Of the two, the alpha subunit plays the leading role in the functioning of the protein. The bundle of alpha helices in the center of the protein position hydrophobic residues at the exterior of the motif, opening a portal through the lipid bilayer. It is inside this transmembrane domain that residues of the alpha subunit coordinate sodium or potassium ions. The large domain on the cytoplasmic side of the bilayer (furthest from beta subunit) floats in solution and is responsible for binding and hydrolyzing ATP. As with many protein enzymes of phosphoester hydrolysis, the phosphate groups are coordinated with a <scene name='Sandbox_60/Drug_in_complex_mg/1'>magnesium cofactor</scene>. The energy released from high-energy phosphate cleavage is transfered via conformational changes to the transmembrane domain, where ions are forced against their concentration gradient. On the extracellular side, the <scene name='Sandbox_60/Drug_in_complex_situated/1'>beta subunit</scene> moves to prevent the dissociation of potassium ions during their entry into the cell. A third gamma subunit (purple/orange), along with the beta subunit, contributes to the anchoring of transmembrane domains to the phospholipid bilayer. | ||
Revision as of 22:07, 4 January 2012
| |||||||||||
Sources
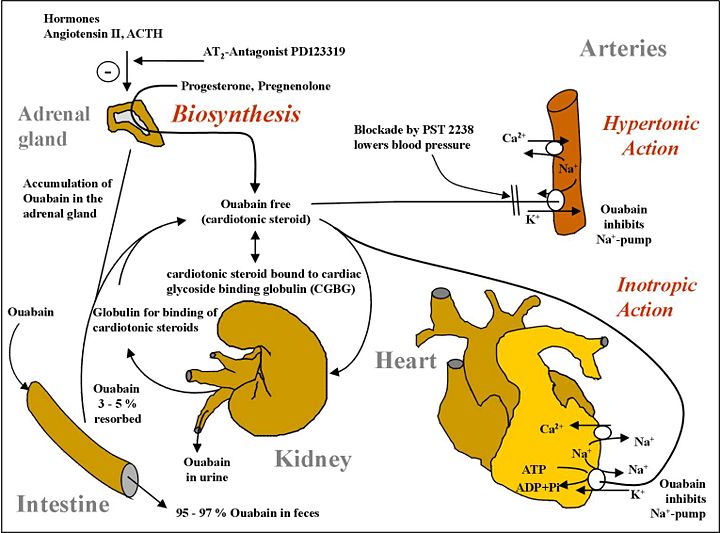
It was recently discovered that ouabain, long thought to be exclusively a plant product, is actually synthesized by animals, and secreted from the adrenal cortex to regulate body osmosis and cellular concentrations of sodium. The image below represents its biosynthesis and metabolism in humans.
Though there are currently synthetic schemes for the production of Ouabain, the compound is usually extracted from the plant sources Strophanthus gratus (left) and Acokanthera schimperi. Somalia is both the native habitat of these plants and the etymological origin of the name ouabain. Somalian tribes have historically used ouabain poisoned arrows for hunting. These arrows are capable of killing a hippopotamus, likely due to cardiac arrest.