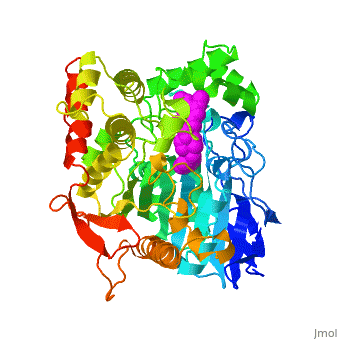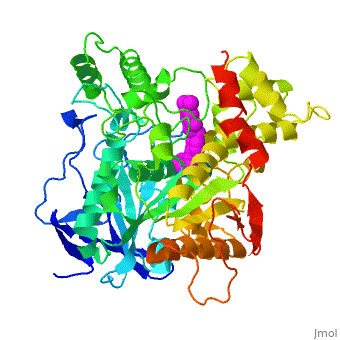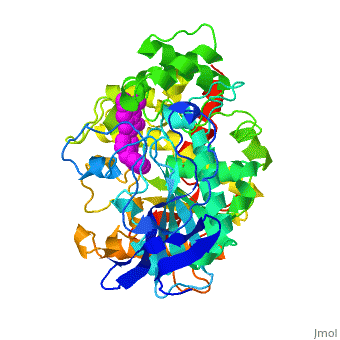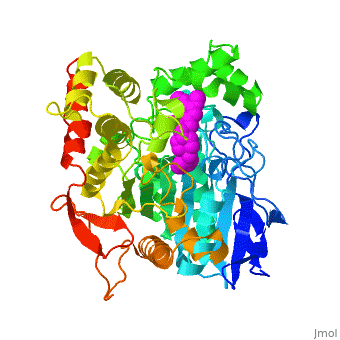Aricept Complexed with Acetylcholinesterase (German)
From Proteopedia
(New page: 250px<br /> {{STRUCTURE_1eve| PDB=1eve | SCENE=Main_Page/E2020_in_ache_spinning/1 }} '''Die dreidimensionale Struktur des anti-Alzh...) |
(→Hintergrund) |
||
| Line 6: | Line 6: | ||
(siehe auch [[AChE bivalent inhibitors (Part II)]]) | (siehe auch [[AChE bivalent inhibitors (Part II)]]) | ||
==Hintergrund== | ==Hintergrund== | ||
| - | Mehrere Cholinesterase Inhibitoren werden entweder bereits zur symptomatischen Behandlung von Alzheimer angewendet oder sind in klinischen Tests. '''E2020''', vermarktet als '''Aricept''', gehoert in die Familie der N-Benzylpiperidin [[acetylcholinesterase]] (AChE) Inhibitoren, entwickelt, produziert und getestet von der Eisai Firma in Japan. Diese Inhibitoren wurden | + | Mehrere Cholinesterase Inhibitoren werden entweder bereits zur symptomatischen Behandlung von Alzheimer angewendet oder sind in klinischen Tests. '''E2020''', vermarktet als '''Aricept''', gehoert in die Familie der N-Benzylpiperidin [[acetylcholinesterase]] (AChE) Inhibitoren, entwickelt, produziert und getestet von der Eisai Firma in Japan. Diese Inhibitoren wurden auf Grund von QSAR Studien entwickelt bevor die 3D Struktur der ''Torpedo californica'' AChE (''Tc''AChE) ([[1ea5]]) bekannt war. Es zeigt guenstige Wirkung in Tiermodellen fuer cholinergische Unterfunktion und hat eine hohe Affinitaet fuer AChE, sowohl von Zitteraal als auch von Maus. |
{{Clear}} | {{Clear}} | ||
<applet load='1eve.pdb' size='500' frame='true' align='right' scene='Main_Page/E2020_in_ache_spinning/1' /> | <applet load='1eve.pdb' size='500' frame='true' align='right' scene='Main_Page/E2020_in_ache_spinning/1' /> | ||
| + | |||
==Results== | ==Results== | ||
The X-ray structure of the E2020-''Tc''AChE complex shows that E2020 has a <scene name='1eve/E2020_close_up_with_84_279/13'>unique orientation</scene> along the active-site gorge, extending from the anionic subsite (<scene name='1eve/E2020_close_up_with_84lbld/7'>W84</scene>) of the active site, at the bottom, to the peripheral anionic site (<scene name='1eve/E2020_close_up_with_84_279lbld/5'>near W279</scene>), at the top, via aromatic stacking interactions with conserved aromatic acid residues. E2020 does not, however, interact directly with either the catalytic triad or the 'oxyanion hole' but only <scene name='1eve/E20_interactionshown/8'>indirectly via solvent molecules</scene>. | The X-ray structure of the E2020-''Tc''AChE complex shows that E2020 has a <scene name='1eve/E2020_close_up_with_84_279/13'>unique orientation</scene> along the active-site gorge, extending from the anionic subsite (<scene name='1eve/E2020_close_up_with_84lbld/7'>W84</scene>) of the active site, at the bottom, to the peripheral anionic site (<scene name='1eve/E2020_close_up_with_84_279lbld/5'>near W279</scene>), at the top, via aromatic stacking interactions with conserved aromatic acid residues. E2020 does not, however, interact directly with either the catalytic triad or the 'oxyanion hole' but only <scene name='1eve/E20_interactionshown/8'>indirectly via solvent molecules</scene>. | ||
Revision as of 00:45, 1 May 2012
| |||||||||
| 1eve, resolution 2.50Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | , | ||||||||
| Activity: | Acetylcholinesterase, with EC number 3.1.1.7 | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
Die dreidimensionale Struktur des anti-Alzheimer Medikaments Aricept in Komplex mit Azetylcholinesterase
(siehe auch AChE bivalent inhibitors (Part II))
Contents |
Hintergrund
Mehrere Cholinesterase Inhibitoren werden entweder bereits zur symptomatischen Behandlung von Alzheimer angewendet oder sind in klinischen Tests. E2020, vermarktet als Aricept, gehoert in die Familie der N-Benzylpiperidin acetylcholinesterase (AChE) Inhibitoren, entwickelt, produziert und getestet von der Eisai Firma in Japan. Diese Inhibitoren wurden auf Grund von QSAR Studien entwickelt bevor die 3D Struktur der Torpedo californica AChE (TcAChE) (1ea5) bekannt war. Es zeigt guenstige Wirkung in Tiermodellen fuer cholinergische Unterfunktion und hat eine hohe Affinitaet fuer AChE, sowohl von Zitteraal als auch von Maus.
|
Results
The X-ray structure of the E2020-TcAChE complex shows that E2020 has a along the active-site gorge, extending from the anionic subsite () of the active site, at the bottom, to the peripheral anionic site (), at the top, via aromatic stacking interactions with conserved aromatic acid residues. E2020 does not, however, interact directly with either the catalytic triad or the 'oxyanion hole' but only .
Conclusions
The X-ray structure shows, a posteriori, that the design of E2020 took advantage of several important features of the active-site gorge of AChE, to produce a drug with both high affinity for AChE and a high degree of selectivity for AChE versus butyrylcholinesterase (BChE). It also delineates voids within the gorge that are not occupied by E2020 and could provide sites for potential modification of E2020 to produce drugs with improved pharmacological profiles.
About this Structure
1EVE is a 1 chain structure with sequence from Torpedo californica. The June 2004 RCSB PDB Molecule of the Month feature on Acetylcholinesterase by David S. Goodsell is 10.2210/rcsb_pdb/mom_2004_6. Full crystallographic information is available from OCA.
Additional Resources
For additional information, see: Alzheimer's Disease
Reference
Structure of acetylcholinesterase complexed with E2020 (Aricept): implications for the design of new anti-Alzheimer drugs., Kryger G, Silman I, Sussman JL, Structure. 1999 Mar 15;7(3):297-307. PMID:10368299
See 1eve (Chinese), 1eve (French), 1eve (Arabic), 1eve (Russian), 1eve (Spanish), & 1eve (Turkish).
Proteopedia Page Contributors and Editors (what is this?)
Karsten Theis, Jaime Prilusky, Alexander Berchansky, Joel L. Sussman