Sandbox Reserved 475
From Proteopedia
| Line 28: | Line 28: | ||
<span style="font-size:110%"> | <span style="font-size:110%"> | ||
| - | Nicotinic Acetylcholine Receptor is composed up '''five distinct subunits''' (shown in figure to left): '''α1''', '''α2''', '''β1''', '''δ''', and '''ε''' at approximately a molecular mass of 290 kDa. The subunits are symmetrically arranged around a central pore. Each subunit is a transmembrane domain with the N-terminus and C-terminus located on the extracellular side of the plasma membrane. Each subunit are composed of long protein chains that extend across the cell membrane. The subunits have a composition of around '''~20%''' <scene name='Sandbox_Reserved_475/My_scene_2/1'>beta sheets</scene> and '''~40%''' <scene name='Sandbox_Reserved_475/My_scene/1'>alpha helices</scene> from about ~370 residues in total.<ref name="Refined"/> | + | Nicotinic Acetylcholine Receptor is composed up '''five distinct subunits''' (shown in figure to left): '''α1''', '''α2''', '''β1''', '''δ''', and '''ε''' at approximately a molecular mass of 290 kDa. The subunits are symmetrically arranged around a central pore. Each subunit is a transmembrane domain with the <scene name='Sandbox_Reserved_475/My_scene_4/2'>N-terminus (in blue)</scene>and<scene name='Sandbox_Reserved_475/My_scene_4/2'>C-terminus (in red)</scene> located on the extracellular side of the plasma membrane. Each subunit are composed of long protein chains that extend across the cell membrane. The subunits have a composition of around '''~20%''' <scene name='Sandbox_Reserved_475/My_scene_2/1'>beta sheets</scene> and '''~40%''' <scene name='Sandbox_Reserved_475/My_scene/1'>alpha helices</scene> from about ~370 residues in total.<ref name="Refined"/> |
</span> | </span> | ||
Revision as of 11:08, 1 May 2012
| This Sandbox is Reserved from 13/03/2012, through 01/06/2012 for use in the course "Proteins and Molecular Mechanisms" taught by Robert B. Rose at the North Carolina State University, Raleigh, NC USA. This reservation includes Sandbox Reserved 451 through Sandbox Reserved 500. | |||||||
To get started:
More help: Help:Editing For more help, look at this link: http://www.proteopedia.org/wiki/index.php/Help:Getting_Started_in_Proteopedia
ACETYLCHOLINE RECEPTOR
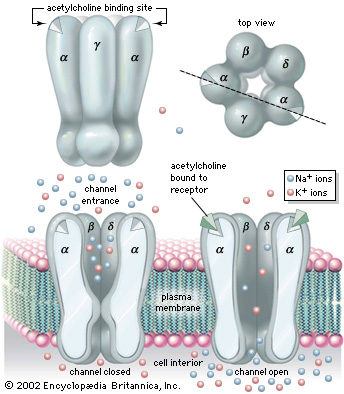
Nicotinic Acetylcholine Receptor (nAChRs) is a neurotransmitter receptor also known as a specialized integral membrane protein. Integral membrane proteins are permanently bound to the phospholipid bilayer. They provide a communication pathway between the internal and external environments of the cell. Acetylcholine receptor proteins reside as ligand-gated ion channels on the motor end plate of the postsynaptic cleft in the neuromuscular junction.[1]
StructureNicotinic Acetylcholine Receptor is composed up five distinct subunits (shown in figure to left): α1, α2, β1, δ, and ε at approximately a molecular mass of 290 kDa. The subunits are symmetrically arranged around a central pore. Each subunit is a transmembrane domain with the and located on the extracellular side of the plasma membrane. Each subunit are composed of long protein chains that extend across the cell membrane. The subunits have a composition of around ~20% and ~40% from about ~370 residues in total.[5]
In vertebrates, nicotinic receptors are classified by their main sites of expression into two subtypes: muscle-type nicotinic receptors and neuronal-type nicotinic receptors. In the muscle-type receptors, found at the neuromuscular junction, receptors are either the embryonic form, composed of α1, β1, δ, and γ subunits in a 2:1:1:1 ratio, or the adult form composed of α1, β1, δ, and ε subunits in a 2:1:1:1 ratio. The neuronal subtypes are various combinations of twelve different nicotinic receptor subunits: α2 through α10 and β2 through β4. In both muscle-type and neuronal-type receptors, the subunits are somewhat similar to one another, especially in the hydrophobic regions.
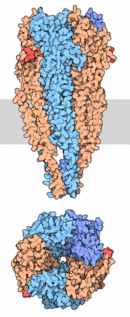
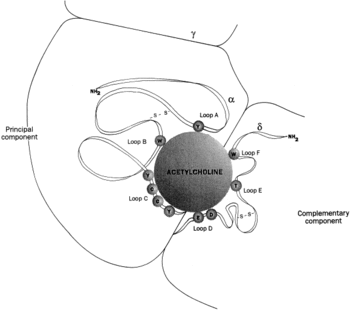
As shown in the picture to the right, rendered using electron microscopy to show the two α-subunits are colored orange in this homeric receptor example. The two α-subunits each contain a ligand binding site colored red. Cys-192 and Cys-193, which are close proximity to the allosteric binding sites on the α-subunits for acetylcholine have been experimentally tested to show that disulfide residues provides stability in the binding of the neurotransmitter.[5] The image (bottom left[6]) shows a simplified depiction of the main components of one of the binding sites in a nAChR. Acetylcholine is shown to interact with various amino acid residues but specifically with the series of amino acids: Tyrosine (Y), Cysteine (C), Cysteine (C), and Tyrosine (Y). The cysteine sulfide residues form the disulfide interaction which aids in acetylcholine binding. The disulfide bonds facilitate the loop formations necessary for the appropriate bonds being formed with acetylcholine.[7]
MechanismThe neuromuscular junction is the location where the neuron activates muscle to contract. This is a step in the excitation-contraction coupling of skeletal muscle.
Medical ApplicationsDrugs and ToxinsnAChRs are the target sites of by many toxins such as those present in snake venom such as α-bungarotoxin.[9] These receptors can also be blocked by curare, hexamethonium as well as neuromuscular blocking agents such as those used in medical anesthesia. Neuromuscular blocking agents bind reversibly to nicotinic receptors in the neuromuscular junction. A popular neurotoxin approved by the FDA was Botox Cosmetic which was used as a treatment for moderate to severe glabellar lines. Botox acts very similarly to the neuromuscular blocking agents in that it binds at the postsynaptic cleft to the receptors of the motor end plate and relaxes the two major muscle contractions in the forehead. Botox Cosmetic (botulinum toxin type A) blocks neuromuscular transmission by binding to acceptor sites on motor nerve terminals, entering the nerve terminals, and inhibiting the release of acetylcholine, resulting in paralysis.[10] This inhibition occurs as the neurotoxin cleaves SNAP-25, a protein integral to the successful docking and release of acetylcholine from vesicles situated within nerve endings. DiseasesMyasthenia gravis[11] is a disease where the body's antibodies actually target the acetylcholine receptors at the motor end plate and prevents correct functionality leading to muscular weakness. In a similar case, Lambert–Eaton myasthenic syndrome[12], is another rare autoimmune disease where the body's antibodies target the voltage-gated calcium channels at the presynaptic site and prevent electric signals from reaching the muscles that are in conjunction with the neuron.
References
|