Sandbox Reserved 482
From Proteopedia
| Line 24: | Line 24: | ||
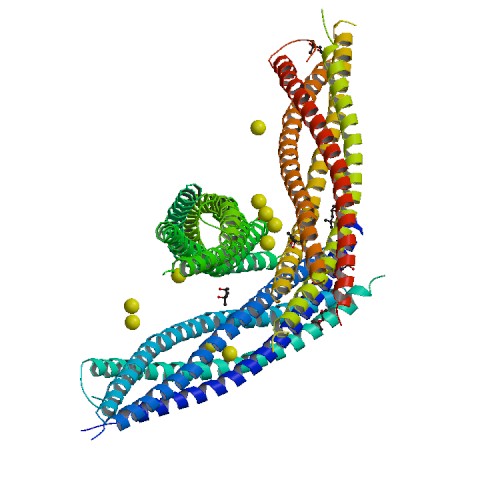
NMR spectroscopy was used to identify a folded N-terminal domain in Syntaxin-1A and to determine its three-dimensional structure. This 120 residue N-terminal domain is conserved in plasma membrane syntaxins. The domain is made up from three long <scene name='Sandbox_Reserved_482/Syntaxin-1a/6'>alpha helices</scene> and an up-and-down bundle with a left-handed twist. Two <scene name='Sandbox_Reserved_482/Syntaxin-1a/7'>ligands</scene> are displayed within the SNARE complex.<ref>Fernandez, I, J Ubach, I Dulubova, et al. "Three-dimensional structure of an evolutionarily conserved N-terminal domain of syntaxin 1A."Cell(Cambridge,Mass.). 94. (1998): 841-849. Web. 2 May. 2012. <http://www.rcsb.org/pdb/explore/explore.do?structureId=1BR0></ref> | NMR spectroscopy was used to identify a folded N-terminal domain in Syntaxin-1A and to determine its three-dimensional structure. This 120 residue N-terminal domain is conserved in plasma membrane syntaxins. The domain is made up from three long <scene name='Sandbox_Reserved_482/Syntaxin-1a/6'>alpha helices</scene> and an up-and-down bundle with a left-handed twist. Two <scene name='Sandbox_Reserved_482/Syntaxin-1a/7'>ligands</scene> are displayed within the SNARE complex.<ref>Fernandez, I, J Ubach, I Dulubova, et al. "Three-dimensional structure of an evolutionarily conserved N-terminal domain of syntaxin 1A."Cell(Cambridge,Mass.). 94. (1998): 841-849. Web. 2 May. 2012. <http://www.rcsb.org/pdb/explore/explore.do?structureId=1BR0></ref> | ||
| - | The image below shows a neuronal synaptic fusion complex to exemplify the use of the <scene name='Sandbox_Reserved_482/Syntaxin-1a/2'>active site</scene>, ASP 68. SNARE proteins are involved in the fusion of vesicles with their target membranes but the structural details of these complexes are still unknown. X-ray is | + | The image below shows a neuronal synaptic fusion complex to exemplify the use of the <scene name='Sandbox_Reserved_482/Syntaxin-1a/2'>active site</scene>, ASP 68. SNARE proteins are involved in the fusion of vesicles with their target membranes but the structural details of these complexes are still unknown. X-ray is used here to show the crystal structure at 2.4 Å resolution of a core synaptic fusion complex containing syntaxin-1A, synaptobrevin-II and SNAP-25B.<ref>Sutton, R.B., D. Fasshauer, R. Jahn, et al. "Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution." Nature. 395 (1998): 347-353. Web. 26 May. 2012. <http://www.rcsb.org/pdb/explore/explore.do?structureId=1sfc></ref> |
[[Image:Syntaxincomplex.jpg]] | [[Image:Syntaxincomplex.jpg]] | ||
Current revision
| This Sandbox is Reserved from 13/03/2012, through 01/06/2012 for use in the course "Proteins and Molecular Mechanisms" taught by Robert B. Rose at the North Carolina State University, Raleigh, NC USA. This reservation includes Sandbox Reserved 451 through Sandbox Reserved 500. | |||||||
To get started:
More help: Help:Editing For more help, look at this link: http://www.proteopedia.org/wiki/index.php/Help:Getting_Started_in_Proteopedia
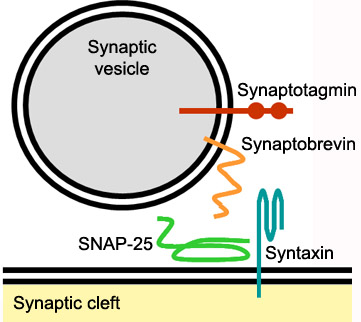
Syntaxin-1AIntroductionSyntaxin-1A is part of the SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) family. It is an integrin plasma membrane protein found almost exclusively in neurons and it is known for its essential roles in neuronal signaling. [1] It assembles in tight core complexes, which promote fusion of carrier vesicles with target compartments. Members of the SNARE class of proteins are expressed in all eukaryotic cells and are distributed in distinct subcellular compartments.
StructureSyntaxin-1A has 288 residues. Residue 1-265 make up the cytoplasmic domain and residue 266-288 form the carboxyl-terminal transmembrane anchor. It adapts a well-folded tertiary structure with repeated forming a colied coil structure.[3]
The image below shows a neuronal synaptic fusion complex to exemplify the use of the , ASP 68. SNARE proteins are involved in the fusion of vesicles with their target membranes but the structural details of these complexes are still unknown. X-ray is used here to show the crystal structure at 2.4 Å resolution of a core synaptic fusion complex containing syntaxin-1A, synaptobrevin-II and SNAP-25B.[5]
Mechanism of actionSyntaxin-1A has an essential role in neuronal signaling. It regulates fusion, docking and trafficking of synaptic vesicles with the plasma membrane. The SNARE complex is a four-alpha-helix bundle composed of syntaxin-1A, synaptobrevin and SNAP-25. In neuronal exocytosis, syntaxin and synaptobrevin are attached in respective membranes by their C-terminal domains, whereas SNAP-25 is connected to the plasma membrane via several cysteine-linked palmitoyl chains. Synaptotagmin acts as a calcium sensor and regulates the SNARE closure. The influx of calcium into the cell triggers the completion of the assembly reaction.
ApplicationsAs mentioned before, the neurodevelopmental disorder Williams syndrome is related to the deletion of, among others, syntaxin-1A. Other typical genes that are deleted are CLIP2, ELN, GTF2I, GTF2IRD1, and LIMK1. The most common symptoms of Williams syndrome are mental disability, heart defects, and unusual facial features.[7]
References
|