Sandbox Reserved 641
From Proteopedia
(→'''Structure''') |
(→'''Mechanism''') |
||
| Line 29: | Line 29: | ||
(3) http://onlinelibrary.wiley.com/doi/10.1002/prot.340120109/pdf | (3) http://onlinelibrary.wiley.com/doi/10.1002/prot.340120109/pdf | ||
(4) http://www.sciencedirect.com/science/article/pii/S0969212699801014 | (4) http://www.sciencedirect.com/science/article/pii/S0969212699801014 | ||
| + | http://www.ncbi.nlm.nih.gov/pmc/articles/PMC283265/?page=2 | ||
Revision as of 01:39, 8 November 2012
| This Sandbox is Reserved from 30/08/2012, through 01/02/2013 for use in the course "Proteins and Molecular Mechanisms" taught by Robert B. Rose at the North Carolina State University, Raleigh, NC USA. This reservation includes Sandbox Reserved 636 through Sandbox Reserved 685. | |||||||
To get started:
More help: Help:Editing For more help, look at this link: http://proteopedia.org/w/Help:Getting_Started_in_Proteopedia
Glutamate Dehydrogenase
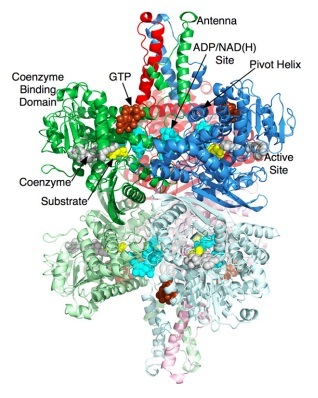
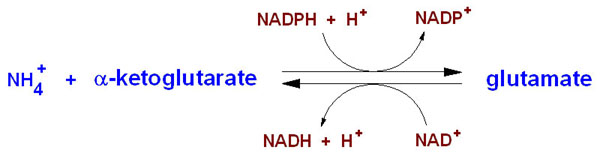
IntroductionGlutamate Dehydrogenase (GDH)is used to remove the ketone group and replace it with an α-amine group on the α-carbon, which forms glutaamte. Glutamate is one of the 20 essential amino acids. This is done in reverse to supply α-ketoglutarate to the tricarboxylic acid (TCA) cycle. GDH is an oxidoreductase, which is an enzyme that transfers electrons from one molecule (reductant/electron donor) to another molecule (oxidant/electron acceptor). StructureGlutamate Dehydrogenase is a hexamer that is comprised of two trimer subunits. These two subunits are stacked on top of each other and composed of three domains. The top of each domain contains a "NAD-binding domain" that has the conserved nucleotide-binding motif. A larger helix-loop-helix structure rises above this and is referred to as an "antenna." This antenna contains approximately 50 amino acids and is thought to play a major role in regulation of the enzyme. This antennae structure is only found in animals. The bottom domain contacts a domain in the other trimer, holding the two trimers together. The total size of each of the subunits is approximately 56.1 kD and 506 amino acids long. When a substrate binds to the enzyme it binds to the deep recess of the cleft between the NAD binding domain and the lower domain. Along the outside surface of the cleft a coenzyme binds causing the binding domain to rotate by about 18 degrees and close down on the substrate and coenzyme. As the cleft is closing the antenna pushes against the pivot helix of the adjacent subunit. The pivot helix rotates counter clockwise around both the helical axis and the trimer 3-fold axis. The hexamer then compresses the inner core showing that catalysis involves the entire hexamer. MechanismNH4+ + α-ketoglutarate + NADPH + 2 H+ → glutamate + NADP+ + H2O Glutamate dehydrogenase is important in nitrogen and glutamate metabolism and energy homeostasis. In the reaction above the forward reaction is essential in converting free ammonia and α-ketoglutarate to glutamate, an amino acid that is used for protein synthesis. The reverse reaction is key reaction that links amino acid metabolism with the Tricarboxylic Acid cycle (TCA cycle). Both reactions utilize nicotinamide nucleotide cofactors: NAD+ when nitrogen is released and NADPH when nitrogen is used. Glutamate dehydrogenase is regulated by cell energy charge. This requires Adenosine triphosphate (ATP) and Guanosine triphosphate (GTP) are positive allosteric effectors for the forward reaction and Adenosine diphosphate (ADP) and Guanosine diphosphate are positive allosteric effectors for the reverse reaction. When the level of ATP is high, conversion of glutamate to α-ketoglurate and other TCA cycle intermediates is limited; when the cellular energy charge is low, glutamate is converted to ammonia and oxidizable TCA cycle intermediates. Glutamate is an important amino acid since it gives an amine group for many transamination reactions, thus, glutamate dehydrogenase is essential in producing this amino acid. (1) http://www.sciencedirect.com.prox.lib.ncsu.edu/science/article/pii/S0968000408001898 (2) http://onlinelibrary.wiley.com/doi/10.1111/j.1432-1033.1974.tb03565.x/pdf (3) http://onlinelibrary.wiley.com/doi/10.1002/prot.340120109/pdf (4) http://www.sciencedirect.com/science/article/pii/S0969212699801014x/pdf (3) http://onlinelibrary.wiley.com/doi/10.1002/prot.340120109/pdf (4) http://www.sciencedirect.com/science/article/pii/S0969212699801014 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC283265/?page=2 |