Sandbox Reserved 648
From Proteopedia
| Line 10: | Line 10: | ||
==Introduction== | ==Introduction== | ||
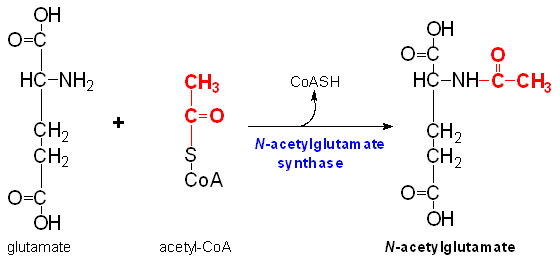
| - | '''N-Acetylglutamate synthase''' (NAGS) is a mitochondrial enzyme involved in the [http://en.wikipedia.org/wiki/Urea_cycle Urea Cycle]. | + | '''N-Acetylglutamate synthase''' (NAGS) is a mitochondrial enzyme involved in the [http://en.wikipedia.org/wiki/Urea_cycle Urea Cycle]. The urea cycle is responsible for producing urea from toxic ammonium. NAGS is most directly used in the conversion of glutamate ([http://en.wikipedia.org/wiki/Glutamate glutamic acid]) and [http://en.wikipedia.org/wiki/CoA Coenzyme A] into N-Acetylglutamate (NAG). N-Acetylglutamate synthase was first discovered as a mammalian liver enzyme but has very low rate of conservation across phyla. |
| - | In mammals, N-Acetylglutamate synthase modulates [http://en.wikipedia.org/wiki/Carbamoyl_phosphate_synthetase_I Carbamoyl phosphate synthetase I] activity. Carbamoyl phosphate synthetase is the first rate limiting enzyme | + | In mammals, N-Acetylglutamate synthase produces the enzyme cofactor N-Acetylglutamate which modulates [http://en.wikipedia.org/wiki/Carbamoyl_phosphate_synthetase_I Carbamoyl phosphate synthetase I] activity. Carbamoyl phosphate synthetase is the first rate limiting enzyme in the Urea cycle. L-arginine, ammonium, and nutrients up-taken through the diet can regulate NAG production through NAGS. L-argninie up regulates the activity of NAGS in mammals. Between vertebral species, 197 amino acids are identical in the current NAGS alignment. In humans, NAGS is synthesized by 534 amino acids that make up a pre-protein but between humans and mice this sequence is only 63% identical. |
Unlike mammalian NAGS, in bacteria and fungi NAGS is inhibited by arginine. | Unlike mammalian NAGS, in bacteria and fungi NAGS is inhibited by arginine. | ||
| Line 39: | Line 39: | ||
==References== | ==References== | ||
| + | |||
| + | Heibel, Sandra Kirsch . "PLOS." PLOS. 7.2 (2012): n. page. Web. 11 Nov. 2012. <http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3287996/>. | ||
Morizono, Hiroki, et. al. "Molecular Genetics and Metabolism." Molecular Genetics and Metabolism. 81.April (2004): 4-11. Web. 11 Nov. 2012. <http://www.sciencedirect.com.prox.lib.ncsu.edu/science/article/pii/S1096719204000046>. | Morizono, Hiroki, et. al. "Molecular Genetics and Metabolism." Molecular Genetics and Metabolism. 81.April (2004): 4-11. Web. 11 Nov. 2012. <http://www.sciencedirect.com.prox.lib.ncsu.edu/science/article/pii/S1096719204000046>. | ||
===Footnotes=== | ===Footnotes=== | ||
| + | |||
{{reflist}} | {{reflist}} | ||
Revision as of 01:15, 12 November 2012
| This Sandbox is Reserved from 30/08/2012, through 01/02/2013 for use in the course "Proteins and Molecular Mechanisms" taught by Robert B. Rose at the North Carolina State University, Raleigh, NC USA. This reservation includes Sandbox Reserved 636 through Sandbox Reserved 685. | |||||||
To get started:
More help: Help:Editing For more help, look at this link: http://proteopedia.org/w/Help:Getting_Started_in_Proteopedia
N-Acetylglutamate synthase
IntroductionN-Acetylglutamate synthase (NAGS) is a mitochondrial enzyme involved in the Urea Cycle. The urea cycle is responsible for producing urea from toxic ammonium. NAGS is most directly used in the conversion of glutamate (glutamic acid) and Coenzyme A into N-Acetylglutamate (NAG). N-Acetylglutamate synthase was first discovered as a mammalian liver enzyme but has very low rate of conservation across phyla. In mammals, N-Acetylglutamate synthase produces the enzyme cofactor N-Acetylglutamate which modulates Carbamoyl phosphate synthetase I activity. Carbamoyl phosphate synthetase is the first rate limiting enzyme in the Urea cycle. L-arginine, ammonium, and nutrients up-taken through the diet can regulate NAG production through NAGS. L-argninie up regulates the activity of NAGS in mammals. Between vertebral species, 197 amino acids are identical in the current NAGS alignment. In humans, NAGS is synthesized by 534 amino acids that make up a pre-protein but between humans and mice this sequence is only 63% identical. Unlike mammalian NAGS, in bacteria and fungi NAGS is inhibited by arginine. Structure
Mechanism of actionImplications or possible applicationBecause of the nature of N-Acetylglutamate synthase, symptoms of NAGS deficiency present similarly to a CPSI deficiency. Both present with elevated plasma ammonia and glutamine, reduced or absent citrulline, and normal urinary orotate [1]. To tell the difference between the two, an enzyme assay with CPSI and NAG is done. Patients with NAGS deficiency will show normal CPS activity. It is possible to treat NAGS deficiencies with a structural analog to NAG. For many patients, N-carbamylglutamate can be used to restore proper CPSI activity. ReferencesHeibel, Sandra Kirsch . "PLOS." PLOS. 7.2 (2012): n. page. Web. 11 Nov. 2012. <http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3287996/>. Morizono, Hiroki, et. al. "Molecular Genetics and Metabolism." Molecular Genetics and Metabolism. 81.April (2004): 4-11. Web. 11 Nov. 2012. <http://www.sciencedirect.com.prox.lib.ncsu.edu/science/article/pii/S1096719204000046>. Footnotes |