We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
JMS/sandbox5
From Proteopedia
(Difference between revisions)
| Line 2: | Line 2: | ||
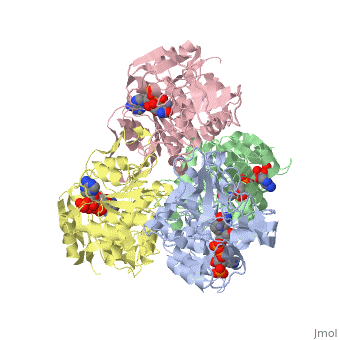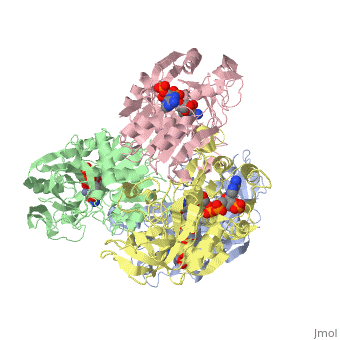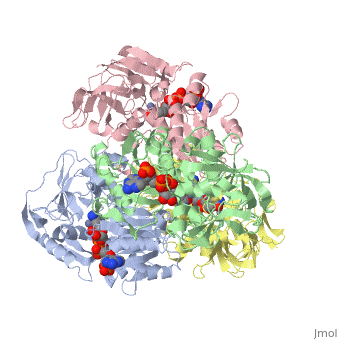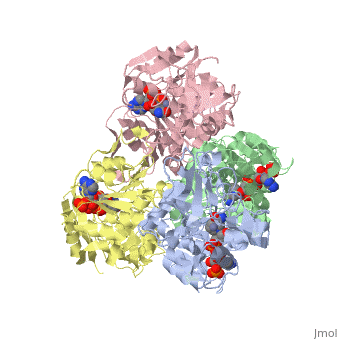
<StructureSection load='1ykf' size='350' side='right' caption='Structure of alcohol dehydrogenase from the thermophile Thermoanaerobacter brockii (PDB entry [[1ykf]])' scene=''> | <StructureSection load='1ykf' size='350' side='right' caption='Structure of alcohol dehydrogenase from the thermophile Thermoanaerobacter brockii (PDB entry [[1ykf]])' scene=''> | ||
Anything in this section will appear adjacent to the 3D structure and will be scrollable. | Anything in this section will appear adjacent to the 3D structure and will be scrollable. | ||
| - | |||
| - | </StructureSection> | ||
TbADH has an extraordinary ability to remain folded in extreme temperatures - up to 95 degrees celsius! How an enzyme can withstand these extreme temperatures depends on enthalpic and entropic strategies, as seen from the GIbb's equation: ∆G = ∆H - T∆S; Where ∆G is negative, the products form spontaneously, which in our context means proteins will fold spontaneously. From the equation is is clear that to maintain a value of ∆G, the enzyme has two available paths. It can decrease ∆H, or it can increase ∆S. TbADH does both. | TbADH has an extraordinary ability to remain folded in extreme temperatures - up to 95 degrees celsius! How an enzyme can withstand these extreme temperatures depends on enthalpic and entropic strategies, as seen from the GIbb's equation: ∆G = ∆H - T∆S; Where ∆G is negative, the products form spontaneously, which in our context means proteins will fold spontaneously. From the equation is is clear that to maintain a value of ∆G, the enzyme has two available paths. It can decrease ∆H, or it can increase ∆S. TbADH does both. | ||
| Line 9: | Line 7: | ||
Through studies like Prof. Burstein's, researchers are gaining insight into how proteins play by the rules of thermodynamics. Any insight into the physics of protein stability has that satisfying feeling that accompanies successful reductive explanation. | Through studies like Prof. Burstein's, researchers are gaining insight into how proteins play by the rules of thermodynamics. Any insight into the physics of protein stability has that satisfying feeling that accompanies successful reductive explanation. | ||
| + | </StructureSection> | ||
Revision as of 14:29, 21 November 2012
Structure
| |||||||||||