We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
JMS/sandbox5
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
== Structure == | == Structure == | ||
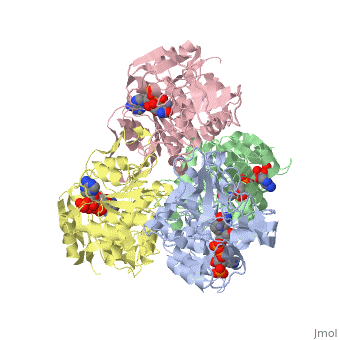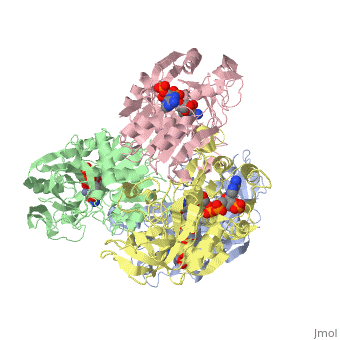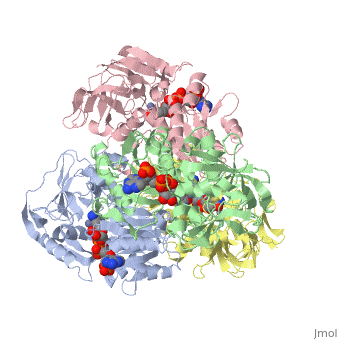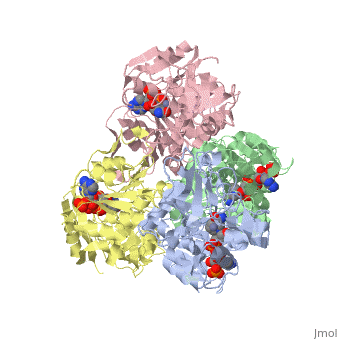
<StructureSection load='1ykf' size='350' side='right' caption='Structure of alcohol dehydrogenase from the thermophile Thermoanaerobacter brockii (PDB entry [[1ykf]])' scene=''> | <StructureSection load='1ykf' size='350' side='right' caption='Structure of alcohol dehydrogenase from the thermophile Thermoanaerobacter brockii (PDB entry [[1ykf]])' scene=''> | ||
| - | Anything in this section will appear adjacent to the 3D structure and will be scrollable. | ||
TbADH has an extraordinary ability to remain folded in extreme temperatures - up to 95 degrees celsius! How an enzyme can withstand these extreme temperatures depends on enthalpic and entropic strategies, as seen from the GIbb's equation: ∆G = ∆H - T∆S; Where ∆G is negative, the products form spontaneously, which in our context means proteins will fold spontaneously. From the equation is is clear that to maintain a value of ∆G, the enzyme has two available paths. It can decrease ∆H, or it can increase ∆S. TbADH does both. | TbADH has an extraordinary ability to remain folded in extreme temperatures - up to 95 degrees celsius! How an enzyme can withstand these extreme temperatures depends on enthalpic and entropic strategies, as seen from the GIbb's equation: ∆G = ∆H - T∆S; Where ∆G is negative, the products form spontaneously, which in our context means proteins will fold spontaneously. From the equation is is clear that to maintain a value of ∆G, the enzyme has two available paths. It can decrease ∆H, or it can increase ∆S. TbADH does both. | ||
Revision as of 14:29, 21 November 2012
Structure
| |||||||||||