VRC01 gp120 complex
From Proteopedia
| Line 1: | Line 1: | ||
| - | < | + | The crystal structure of ToxT is resolved in monomeric form, after isolation from <i>Vibrio cholerae</i> strain O395.<ref name="structure">PMID: 20133655</ref> |
| - | + | ||
==Introduction== | ==Introduction== | ||
| + | <b>ToxT</b> is a molecule at the end of a transcriptional cascade that autoregulates the transcription of the primary virulence factors of <i>Vibrio cholerae</i>[http://en.wikipedia.org/wiki/Vibrio_cholerae] and itself. ToxT is a cytoplasmic protein that is activated in turn by ToxR, which is itself activated by ToxS in response to environmental stimuli.<ref>Kenneth Todar [http://www.textbookofbacteriology.net/cholera.html] ''Vibrio cholerae'' and Asiatic Cholera, Todar's Online Textbook of Bacteriology. Date of access: 2011-11-28.</ref> These two factors, cholera toxin (CT)[http://en.wikipedia.org/wiki/Cholera_toxin] and the toxin co-regulated pilus (TCP), are instrumental in causing the disease <b>cholera</b>[http://en.wikipedia.org/wiki/Cholera]. This is an intestinal infection resulting in massive water loss in the affected individual, causing extreme dehydration.[http://en.wikipedia.org/wiki/Cholera] Rehydration is sufficient as treatment. | ||
| + | <StructureSection load='3SE9' color='structure' size='500' frame='true' align='right' caption='ToxT complex with palmitoleic acid, 1.9 Angstrom resolution crystal structure, [[3gbg]]' > | ||
| - | Caspases (cysteine-aspartic proteases) are proteolytic enzymes that play essential roles in [http://en.wikipedia.org/wiki/Apoptosis apoptosis], development, inflammation, and the immune system. As the name suggests, caspases cleave proteins on the C-side of aspartic acid residues. As of now, around 15 caspases have been identified in two categories, inflammatory (in which caspase-1 is classified) and apoptotic. Apoptotic caspases are further divided into initiator (apical) caspases and effector (executioner) caspases. Initiator caspases cleave and activate effector caspases, which in turn start apoptosis by cleaving other proteins in a cascade reaction. Faulty or premature apoptosis is a main component of autoimmune diseases, leading to research into caspase inhibitors to treat diseases like Alzheimer's. Inflammatory caspases on the other hand usually work by proteolytically activating cytokines, leading to a different kind of cell mediated death called [http://en.wikipedia.org/wiki/Pyroptosis pyroptosis]. | ||
| - | [[image:Caspase-1_Zymogen.jpg|thumb|left|200px|'''Human Caspase-1 Zymogen''']] | ||
| - | == | + | ==Structural Features== |
| + | <u>DNA-binding</u>. ToxT belongs to a family of transcriptional regulators headed by and known as AraC.<ref name="structure">PMID: 20133655</ref> The AraC family is characterized by a 100 amino acid region of sequence similarity that forms a <scene name='ToxT_Transcriptional_Regulator_in_Vibrio_cholerae/Two_hth_domains/1'>DNA-binding domain</scene> with two helix-turn-helix motifs (one on either side of the black linker). <ref name="arac">PMID: 11282467</ref> This DNA binding domain is composed of seven alpha helices. HTH1 is composed of alpha helices five and six, while HTH2 is composed of alpha helices eight and nine. The two HTH regions are linked by the very polar alpha helix seven(shown in black). The overall domain is located at the C-terminus.<ref name="structure">PMID: 20133655</ref> Assuming ToxT is similar in mechanism to other AraC proteins, helix six from HTH1 and helix nine from HTH2 become aligned with the help of helix seven. Helix seven is positioned to attach to the N terminal binding pocket(the polar linking region) to allow binding to major consecutive grooves of target DNA (specific promoters for virulence genes).<ref name="structure">PMID: 20133655</ref>[http://www.pnas.org/content/107/7/2860/F3.large.jpg]. The conformation of helix seven is dependent on the ligand bound. | ||
| + | <br/> | ||
| + | <br/> | ||
| + | <u>Ligand-binding</u>. <scene name='ToxT_Transcriptional_Regulator_in_Vibrio_cholerae/Barrel/1'>A nine-stranded beta sheet sandwich</scene> or "jelly-roll" with three other alpha helices (overall making up the <scene name='ToxT_Transcriptional_Regulator_in_Vibrio_cholerae/N-terminus/1'>N-terminus</scene>) contain a <scene name='ToxT_Transcriptional_Regulator_in_Vibrio_cholerae/Binding_pocket/1'>binding pocket</scene>. This is made from several residues from the N-terminus (Y12, Y20, F22, L25, I27, K31, F33, L61, F69, L71, V81, and V83), and a few from the C-terminus (I226, K230, M259, V261, Y266, and M269). This pocket contains a sixteen-carbon fatty acid positioned in a conformation such that its negatively charged carboxylate group forms salt bridges between K31 of the N-terminal domain, and K230 from the C-terminal domain. The pocket is highly hydrophobic, and has a known volume of 780.9 Angstroms.<ref name="structure">PMID: 20133655</ref> This pocket contains a ligand: <scene name='ToxT_Transcriptional_Regulator_in_Vibrio_cholerae/Binding_pocket/2'>cis-palmitoleate</scene> <ref name="structure">PMID: 20133655</ref> which appears to have a negative effect on virulence when present in vitro. The <i>cis</i>-palmitoleate forms <scene name='ToxT_Transcriptional_Regulator_in_Vibrio_cholerae/Salt_bridges_pam/1'>salt bridges</scene> with residues K31 and K230 (for detail, see Figure 1B of: [http://www.pnas.org/content/107/7/2860/F1.large.jpg]). This unsaturated fatty acid, like other UFAs,[http://en.wikipedia.org/wiki/Fatty_acid#Unsaturated_fatty_acids] tend to inhibit genes under the control of ToxT. | ||
| + | Specifically, the <i>cis</i>-palmitoleate (PAM) appears to change ToxT's conformation, and thus lower its ability to bind DNA and form dimers.<ref name="structure">PMID: 20133655</ref> The presence of UFAs is associated with being in the lumen of the intestine during the bacterial infection. PAM brings K31 and K230 together from either end of the protein, and essentially closes off ToxT. K230 is at the end of helix seven, and binding to K31 causes helix six to be pulled into an unfavorable conformation that deters DNA binding. In lower concentration of fatty acids, ie: after penetrating the intestine's mucus, PAM is in lower concentration. At this point, charge-charge repulsion between K31 and K230 leads to a destabilization of the closed conformation of ToxT. This repulsion prompts the opening of the N and C terminal domains. The freedom of helices six and seven to find a favorable configuration allows DNA binding to occur.<ref name="structure">PMID: 20133655</ref> | ||
| + | <br/> [[Image:MSA.png|center|300px|thumb| MSA [[1xtc]]]] | ||
| + | <br/> | ||
| + | <u>Dimerization</u>. Though the structure shown is a monomer with two overall domains (N-terminal and C-terminal), ToxT tends to form a dimer.<ref name="dimerization">PMID: 21415495 | ||
| + | </ref> The preferred state of ToxT varies between promoters, but binding to the <i>ctx</i> promoter to generate cholera toxin appears to be possible only in the dimer form.<ref name="virstatin">PMID:17283330</ref>ToxT binds to thirteen base pair sequences (can be single, direct, or inverted repeats) called toxboxes in order to activate their respective promoters.[http://www.sigwiki.info/wiki/Signature:ToxBox] | ||
| + | ==Ligand== | ||
| + | In this resolved structure, <scene name='ToxT_Transcriptional_Regulator_in_Vibrio_cholerae/Pam/2'>cis-palmitoleate</scene>[http://www.ebi.ac.uk/thornton-srv/databases/cgi-bin/pdbsum/GetPage.pl?pdbcode=3gbg&template=ligands.html&l=1.1] is shown, which can be bound in the beta sheet barrel (as discussed above). This unsaturated fatty acid reduces virulence expression in <i>Vibrio cholerae</i>. | ||
| + | </StructureSection> | ||
| - | <scene name='Human_Caspase-1/Caspase-1/1'>Caspase-1</scene> begins as a [http://en.wikipedia.org/wiki/Zymogen zymogen], with its two subunits and a CARD prodomain all in one peptide sequence. Activation is thought to be initiated by removal of the [http://en.wikipedia.org/wiki/CARD_domain CARD domain] and dimerization. Bonds between subunits can be cleaved, leading to the maturation of the enzyme, but this does not necessarily convey greater activity. ASC and Ipaf have been identified as possible regulators of caspase-1 in a structure called the inflammasome. ASC leads to ATP-driven activation of caspase-1, while Ipaf connects signals triggered by intracellular pathogens.<ref name=Mariathasan>PMID:15190255</ref> Both of these activators are essential for caspase-1 driven cell death, showing a link between inflammation and apoptosis. Adaptors in the inflammasome react to extracellular and endogenous danger signals, usually flaggelin, to activate caspase-1 and begin the inflammatory response.<ref name=Franchi>PMID:19221555</ref> | ||
| - | == | + | ==Further Study== |
| + | Conclusive results about what activates ToxT itself has not yet been found. The varying activity of ToxT dependent on the presence of <i>cis</i>-palmitoleate or other unsaturated fatty acids represents a detailed method of effective pathogenicity in humans, but may not be a reasonable target for drug treatment. By restricting transcription (and thus translation and protein production) of virulence genes until the bacterium is determined to be in a favorable location for infection, <i>Vibrio cholerae</i> avoids wasting energy producing virulence factors that will just be cleared by the intestine. This is a specific mechanism to ensure that the bacterium also injects CT and TCP where they will do the most damage, perpetuating the infection. <ref>Kenneth Todar [http://www.textbookofbacteriology.net/cholera.html] ''Vibrio cholerae'' and Asiatic Cholera, Todar's Online Textbook of Bacteriology. Date of access: 2011-11-28.</ref> Despite the lack of information about what activates ToxT itself, it is understood that the transcription of ctxA and tcpA by vibrio cholerae is sharply reduced in the presence of oleic, linoleic acid, and arachidonic acid, all of which are components of bile. Therefore, one may hypothesize that it may be possible to use the structure of a UFA or SFA to design a small molecule inhibitor of ToxT which may be used to treat or prevent cholera. | ||
| - | Caspase-1 consists of four subunits, two <scene name='Human_Caspase-1/Alpha_subunit/1'>20kDa (alpha)</scene> and two <scene name='Human_Caspase-1/Beta_subunit/1'>10kDa (beta)</scene> pieces, joining to make a homodimer. It has two active sites, which can be linked as is the case with caspase-1. The large alpha subunit contains the proteolytic dyad residues <scene name='Human_Caspase-1/Cys285_and_his_237/1'>Cys285 and His237</scene>, while the smaller subunit contains residues that form a groove for ligand binding. A '<scene name='Human_Caspase-1/Asp_socket/2'>socket</scene>' composed of Arg179, Gln283, and Arg341 holds the carboxylate side chain of the Asp residue tightly in place. | ||
| - | == | + | ==Evolution== |
| + | Vibrio cholerae is a highly diverse species in which some strains are completely harmless, whereas other strains have the capacity to cause global cholera pandemics. It has been discovered that in each epidemic and pandemic strain, there is a chromosomal pathogenicity island (PAI) that is not present in the nonpathogenic strains. The region containing two ToxR-regulated genes (aldA and tagA) is composed of 13kb of previously unidentified DNA. <ref>Bailey, Camella "A Vibrio cholerae pathogenicity island associated with epidemic and pademic strains" (1997).</ref>This region is part of a PAI that contains ToxT and a gene cluster a critical colonization factor and TCP. The PAI is 39.5 kb long, contains putative integrase and transposase genes, and inserts near a 10Sa RNA gene. One may infer that the PAI could have originated from a bacteriophage. This PAI was also found in two non-O1/non-O139 (which are both pandemic) sero type strains. Therefore, one may hypothesize that the PAI could be transferred within other strains of Vibrio cholerae. | ||
| - | + | [[Image:centroid.png|center|300px|thumb| Centroid RNA [[1xtc]]]] | |
| - | + | ==RNA Structure== | |
| + | Here we have the centroid structure of the mRNA of ToxT. This mRNA is shown in its most stable conformation, with the less stable, higher energy regions in red. The lighter colored regions are more stable and lower in energy. | ||
| - | == | + | ==References== |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
Revision as of 01:43, 26 November 2012
The crystal structure of ToxT is resolved in monomeric form, after isolation from Vibrio cholerae strain O395.[1]
Contents |
Introduction
ToxT is a molecule at the end of a transcriptional cascade that autoregulates the transcription of the primary virulence factors of Vibrio cholerae[1] and itself. ToxT is a cytoplasmic protein that is activated in turn by ToxR, which is itself activated by ToxS in response to environmental stimuli.[2] These two factors, cholera toxin (CT)[2] and the toxin co-regulated pilus (TCP), are instrumental in causing the disease cholera[3]. This is an intestinal infection resulting in massive water loss in the affected individual, causing extreme dehydration.[4] Rehydration is sufficient as treatment.
| |||||||||||
Further Study
Conclusive results about what activates ToxT itself has not yet been found. The varying activity of ToxT dependent on the presence of cis-palmitoleate or other unsaturated fatty acids represents a detailed method of effective pathogenicity in humans, but may not be a reasonable target for drug treatment. By restricting transcription (and thus translation and protein production) of virulence genes until the bacterium is determined to be in a favorable location for infection, Vibrio cholerae avoids wasting energy producing virulence factors that will just be cleared by the intestine. This is a specific mechanism to ensure that the bacterium also injects CT and TCP where they will do the most damage, perpetuating the infection. [6] Despite the lack of information about what activates ToxT itself, it is understood that the transcription of ctxA and tcpA by vibrio cholerae is sharply reduced in the presence of oleic, linoleic acid, and arachidonic acid, all of which are components of bile. Therefore, one may hypothesize that it may be possible to use the structure of a UFA or SFA to design a small molecule inhibitor of ToxT which may be used to treat or prevent cholera.
Evolution
Vibrio cholerae is a highly diverse species in which some strains are completely harmless, whereas other strains have the capacity to cause global cholera pandemics. It has been discovered that in each epidemic and pandemic strain, there is a chromosomal pathogenicity island (PAI) that is not present in the nonpathogenic strains. The region containing two ToxR-regulated genes (aldA and tagA) is composed of 13kb of previously unidentified DNA. [7]This region is part of a PAI that contains ToxT and a gene cluster a critical colonization factor and TCP. The PAI is 39.5 kb long, contains putative integrase and transposase genes, and inserts near a 10Sa RNA gene. One may infer that the PAI could have originated from a bacteriophage. This PAI was also found in two non-O1/non-O139 (which are both pandemic) sero type strains. Therefore, one may hypothesize that the PAI could be transferred within other strains of Vibrio cholerae.
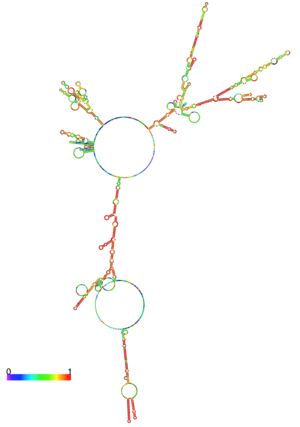
RNA Structure
Here we have the centroid structure of the mRNA of ToxT. This mRNA is shown in its most stable conformation, with the less stable, higher energy regions in red. The lighter colored regions are more stable and lower in energy.
References
Proteopedia Page Contributors and Editors (what is this?)
Amanda Valdiosera, Michal Harel, Chris Casey, Alexander Berchansky

