We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox 645
From Proteopedia
(Difference between revisions)
| Line 21: | Line 21: | ||
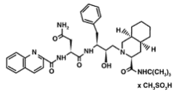
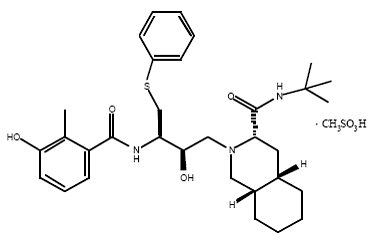
1) Saquinavir (Invirase) is known to be one of the first FDA approved protease inhibitor for HIV treatment. This usually occurs by HIV protease binding an active site tunnel tightly, which will prevent polyproteins from also binding. HIV’s chemical structure has the ability to mimic the tetrahedral intermediate of the hydrolytic reaction to interact strong with the catalytic Asp residues. Knowing that, Saquinavir is an uncleavable ligand by studying its similar conformational changes in binding saquinavir or a polypeptide. ((http://www.rxlist.com/invirase-drug.htm)))) | 1) Saquinavir (Invirase) is known to be one of the first FDA approved protease inhibitor for HIV treatment. This usually occurs by HIV protease binding an active site tunnel tightly, which will prevent polyproteins from also binding. HIV’s chemical structure has the ability to mimic the tetrahedral intermediate of the hydrolytic reaction to interact strong with the catalytic Asp residues. Knowing that, Saquinavir is an uncleavable ligand by studying its similar conformational changes in binding saquinavir or a polypeptide. ((http://www.rxlist.com/invirase-drug.htm)))) | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| Line 27: | Line 35: | ||
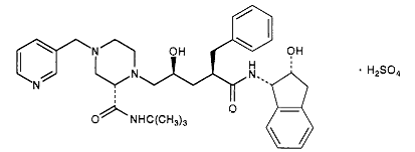
2) Indinavir (Crixivan) this protease inhibitor is an oral medication that blocks the protease activity and leads to defect the viruses. Once these viruses are unable to reach the body’s cells, it will decrease in number. This inhibitor doesn’t have the ability to prevent the HIV transmission among individuals, nor does it cure AIDS or HIV infection. Indinavir is approved by the FDA in March 1995. ((((http://www.medicinenet.com/indinavir/article.htm ))) | 2) Indinavir (Crixivan) this protease inhibitor is an oral medication that blocks the protease activity and leads to defect the viruses. Once these viruses are unable to reach the body’s cells, it will decrease in number. This inhibitor doesn’t have the ability to prevent the HIV transmission among individuals, nor does it cure AIDS or HIV infection. Indinavir is approved by the FDA in March 1995. ((((http://www.medicinenet.com/indinavir/article.htm ))) | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| Line 35: | Line 57: | ||
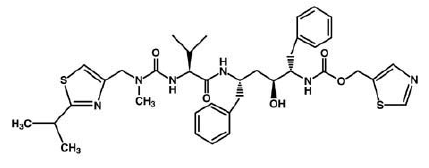
3) Ritonavir (Norvir), has the same effect of indinavir on HIV protease. It was approved by the FDA in June 1999. This drug consists of capsules or tablets 100 mg; Solution: 80 mg/ml. It is recommended that adults consume 600 mg twice a day and then increase by 100 mg in every 2 days. Ritonavir can interact with many drugs leading those drugs to increase or decrease in effectiveness. It increases the concentration of Mycobutin and Viagra; however, decrease the concentration of Demerol. ((((http://www.medicinenet.com/ritonavir/article.htm)))) | 3) Ritonavir (Norvir), has the same effect of indinavir on HIV protease. It was approved by the FDA in June 1999. This drug consists of capsules or tablets 100 mg; Solution: 80 mg/ml. It is recommended that adults consume 600 mg twice a day and then increase by 100 mg in every 2 days. Ritonavir can interact with many drugs leading those drugs to increase or decrease in effectiveness. It increases the concentration of Mycobutin and Viagra; however, decrease the concentration of Demerol. ((((http://www.medicinenet.com/ritonavir/article.htm)))) | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
Revision as of 14:49, 26 November 2012
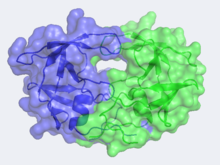
HIV-1 Protease
| |||||||||||