Sandbox reserved 659
From Proteopedia
| Line 7: | Line 7: | ||
<StructureSection load='1B8E' size='350' side='right' caption='Structure of Beta Lactoglobulin (PDB entry [[1B8E]])' scene=''> | <StructureSection load='1B8E' size='350' side='right' caption='Structure of Beta Lactoglobulin (PDB entry [[1B8E]])' scene=''> | ||
| - | Anything in this section will appear adjacent to the 3D structure and will be scrollable. | ||
| - | |||
| - | </StructureSection> | ||
| - | |||
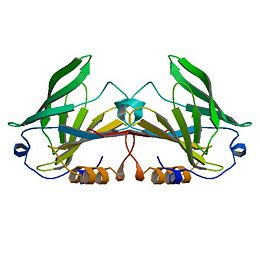
β-Lactoglobulin is a small protein, soluble in dilute salt solution as befits a globulin, with 162 amino acid residues (Mr ∼18,400) that fold up into an 8-stranded, antiparallel β-barrel with a 3-turn α-helix on the outer surface and a ninth β-strand flanking the first strand (see Figure 1). It is this strand that forms a significant part of the dimer interface in the bovine and ovine proteins but, while still present in porcine β-LG, is not involved in the formation of the dimer that forms at low pH. The genetic variants referred to above in the ruminant species result in relatively minor amino acid differences, but the processing properties appear to be significantly affected even by these small changes, such that consideration has been given to removing the less favorable variants by selective breeding (Hill et al., 1997; Harris, 1997). The so-called calyx, or β-barrel, is conical and is made of β-strands A-D forming one sheet, and strands E-H forming a second. Strand A bends through a right angle such that the C-terminal end forms an antiparallel strand with H; strands D and E also form a less significant interaction completely closing the calyx. It is this central cavity, the calyx, that provides the ligand-binding site. On the outer surface of the β-barrel, between strands G and H, is the 3-turn α-helix. The loops that connect the β-strands at the closed end of the calyx, BC, DE, and FG are generally quite short, whereas those at the open end are significantly longer and more flexible. | β-Lactoglobulin is a small protein, soluble in dilute salt solution as befits a globulin, with 162 amino acid residues (Mr ∼18,400) that fold up into an 8-stranded, antiparallel β-barrel with a 3-turn α-helix on the outer surface and a ninth β-strand flanking the first strand (see Figure 1). It is this strand that forms a significant part of the dimer interface in the bovine and ovine proteins but, while still present in porcine β-LG, is not involved in the formation of the dimer that forms at low pH. The genetic variants referred to above in the ruminant species result in relatively minor amino acid differences, but the processing properties appear to be significantly affected even by these small changes, such that consideration has been given to removing the less favorable variants by selective breeding (Hill et al., 1997; Harris, 1997). The so-called calyx, or β-barrel, is conical and is made of β-strands A-D forming one sheet, and strands E-H forming a second. Strand A bends through a right angle such that the C-terminal end forms an antiparallel strand with H; strands D and E also form a less significant interaction completely closing the calyx. It is this central cavity, the calyx, that provides the ligand-binding site. On the outer surface of the β-barrel, between strands G and H, is the 3-turn α-helix. The loops that connect the β-strands at the closed end of the calyx, BC, DE, and FG are generally quite short, whereas those at the open end are significantly longer and more flexible. | ||
| Line 20: | Line 16: | ||
The structure of β-LG has also been determined by NMR techniques by several groups (Belloque and Smith, 1998; Fogolari et al., 1998; Kuwata et al., 1998; Uhrinova et al., 2000) and includes the equine structure (Kobayashi et al., 2000). The bovine protein is monomeric at pH values below 3, and the structure has been determined at this pH. Although there is no matching crystal structure at this pH where flat hexagonal plates of poor diffraction quality can be grown, the structure of the core of the protein is well conserved. However, significant differences do exist, and these have been critically reviewed (Jameson et al., 2002); it is not surprising that it is the external loops that contain these major differences. | The structure of β-LG has also been determined by NMR techniques by several groups (Belloque and Smith, 1998; Fogolari et al., 1998; Kuwata et al., 1998; Uhrinova et al., 2000) and includes the equine structure (Kobayashi et al., 2000). The bovine protein is monomeric at pH values below 3, and the structure has been determined at this pH. Although there is no matching crystal structure at this pH where flat hexagonal plates of poor diffraction quality can be grown, the structure of the core of the protein is well conserved. However, significant differences do exist, and these have been critically reviewed (Jameson et al., 2002); it is not surprising that it is the external loops that contain these major differences. | ||
| + | |||
| + | </Pysiological Purpose> | ||
| + | |||
| + | |||
| + | No definite physiological function has been ascribed to β-LG, although several suggestions have been made, the more compelling of which favor a role in molecular transport or, possibly, as some form of modulator (Kontopidis et al., 2002). Most suggestions concerning the function, understandably, have concentrated on either the lactating cell or more usually the neonate, and a transporter role seems reasonable, since many lipocalins are transporters. β-Lactoglobulin binds both fatty acids and retinol, and the structure is similar to the known transporter, plasma RBP. The nature of the ligand transported, apart from being generally hydrophobic, is not clear, however. Fatty acids, rather than retinal, are found as endogenous ligands in milk, but not all species have a β-LG that binds fatty acids (Perez et al., 1989, 1993), and it seems improbable that the true function will vary from species to species. Similarly, retinol is significantly more soluble in the fat phase of milk and thus will probably be transferred from mother to offspring by that route. A signaling or activity-modulator role appears to be less likely, not just because of the paucity of similar roles reported for other lipocalins (Flower et al., 2000), but also because the data supporting these various activities appear rather circumstantial. Further, for such an important role, one might expect the presence of β-LG in the milks of all species, not just some. What does not appear to have been considered in detail until recently (Kontopidis et al., 2002) is that the function is directly related to maternal physiology. | ||
| + | |||
| + | When the species variation of β-LG sequences is examined along with that of the other lipocalins, what emerges is a family tree like that shown in Figure 4. The RBP are clearly distinct from the lactoglobulins, but there is one protein present in the endometrium, now called glycodelin (formerly PP14, inter alia), that is the closest relative to the β-LG-II gene product (Halttunen et al., 2000; Seppälä, 2002). There are also reports of RBP expressed in the endometrium of cow (Thomas et al., 1992; MacKenzie et al., 1997). Notice also that there is ruminant pseudo-gene identified in both cow and goat that is also most nearly related to the sequence of β-LG-II. Might it be that the protein glycodelin reflects the true, original function of β-LG as a protein involved in some aspect of fetal development in all mammals? In many species, the gene has undergone a gene duplication event and is expressed during lactation for nutritional purposes. More recently, some species, like rodents, lagomorphs, and man, have lost the function of one of these genes through formation of a pseudo-gene, resulting in the gene ceasing to be expressed. The biological properties of the milk protein, such as ligand binding and inhibiting harmful bacterial adhesion in the intestine (Ouwehand et al., 1997) that have been identified, are clearly useful to each species but are subsidiary to those of neonatal nutrition. On the other hand, it is known that low levels of β-LG are expressed in the cow throughout the dry period in a manner that is distinct from α-LA and the caseins (Aslam et al., 1994). Whether this is indicative of another more physiological role for β-LG in the changes that occur to the mammary gland during this period remains unclear at present. | ||
Revision as of 17:29, 26 November 2012
Beta Lactoglobulin
β-Lactoglobulin(β-LG) is the major whey protein of ruminant species and is also present in the milks of many, but not all, other species. Its amino-acid sequence and 3-dimensional structure show that it is a lipocalin, a widely diverse family, most of which bind small hydrophobic ligands and thus may act as specific transporters, as does serum retinol binding protein. Bovine β-LG binds a wide range of ligands, but this may not be the reason for its presence in milk. In reviewing the structure and physicochemical properties of the protein, we present the structures of the ligands cholesterol (at a resolution of 2.0Å, R = 0.221; Rfree = 0.295) and vitamin D2 (at a resolution of 2.4Å, R = 0.212; Rfree = 0.297) each bound to the central binding cavity of bovine β-LG at pH 7.3. Neither ligand is fully visible in the electron density maps, and the less well-ordered regions are the polar end groups at the mouth of the binding site. In a separate experiment, a mercury ion was bound to the free Cys121 (at a resolution of 2.2Å, R = 0.218; Rfree = 0.288) in a way that transmitted a small structural change through Asp137 via Arg148 to the dimer interface. It is not clear if the known dissociation that arises from the reaction of β-LG with HgCl2 results from this perturbation.
In reviewing the structural studies that reveal the ligand binding sites for long-chain fatty acids, retinoids, and steroids, only the central location, common to all lipocalins so far examined, is occupied under the conditions used. We find that there is no crystallographic evidence of another ligand binding site in our crystals grown in approximately 1.3 M citrate, although low ionic strength studies in solution indicate the possible presence of at least one other low affinity site. The apparent ability of the binding site to accommodate a wide range of ligands may point to a possible physiological function. However, by considering the lipocalin family in general, and the species distribution of β-LG in particular, some speculation as to the physiological function can be made. β-Lactoglobulin has been reported as being implicated, inter alia, in hydrophobic ligand transport and uptake, enzyme regulation, and the neonatal acquisition of passive immunity. However, these functions do not appear to be consistent between species. Sequence comparisons among members of the lipocalin family reveal that glycodelin, found in the human endometrium during early pregnancy, is the most closely related to β-LG. Although the function of glycodelin is also unknown, it appears to have effects on the immune system and/or to be involved in differentiation. It is proposed that β-LG, over-expressed in the lactating mammary gland of many, but not all, species, is primarily an important source of amino acids for the offspring of those animals that produce it, but that this function arose by gene duplication from the physiologically essential glycodelin. The other functions that have been associated with β-LG in the neonate are, therefore, fortuitous.
| |||||||||||