Sandbox reserved 659
From Proteopedia
Contents |
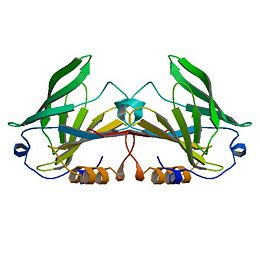
Beta Lactoglobulin
β-Lactoglobulin(β-LG) is the bulk constituent of whey protein in most ruminant species and is also present in the milks of most mammalian species, although not in rodent, human, and lagomorph milks. It has been studied extensively for some time ( Tilley, 1960; Lyster, 1972; Kinsella and Whitehead, 1987; Hambling et al., 1992; Sawyer, 2003). β-LG is a lipocalin as is seen through its amino-acid sequence and structure (Flower et al., 2000). Lipocalins are a varied family which bind small hydrophobic ligands, and often act as transporters in vivo. The purpose of β-LG, however, is not clear. Genetic variants of β-LG expressing genes have been reported, especially in cows and several nonruminant species (Kontopidis, 2004).
Whey is an often used food ingredient in the food industry and has many useful functional characteristics including foaming, gelling, thermal stability, emulsification, and protein fortification. It has also been suggested that it has many useful health benefits including exercise recovery, weight management, cardiovascular health, anti-cancer effects, anti-infection activity, wound repair, and infant nutrition. Human consumption of whey ingredients has steadily increased and has become more and more important to the dairy industry. An issue with whey ingredients is the subtle off flavor often associated with whey proteins. During the Cheddar cheese making process, norbixin leaches into the whey during the draining of the curd. Norbixin, a carotenoid extracted from the bixa orellana seed and added to cheese milk to give Cheddar its expected yellow flavor, would also contribute yellow color to the final product into which the whey ingredient is added. To prevent discoloration of the final product, whey is bleached by either hydrogen peroxide or benzoyl peroxide. In addition to a white color, this also causes lipid oxidation and associated off flavors.
| |||||||||||
Physiological Function of β-LG
| |||||||||||
Tanford Transition of β-LG
| |||||||||||
Implications
For the dairy industry, functionality and nutritional value are the primary issues when dealing with dairy proteins such as beta-lactoglobulin. The genetic variants referred to above in the ruminant species result in relatively minor amino acid differences, but the processing properties appear to be significantly affected even by these small changes, such that consideration has been given to removing the less favorable variants by selective breeding (Hill et al., 1997; Harris, 1997, Kontopidis et al., 2004).
References
G. Kontopidis, C. Holt, L. Sawyer. The ligand binding site of bovine β-lactoglobulin—Evidence for a function? J. Mol. Biol., 318 (2002), pp. 1043–1055
J.P. Hill, M. Boland, A.F. Smith. The effect of β-lactoglobulin variants on milk powder manufacture and properties J.P. Hill, M. Boland (Eds.), Milk Protein Polymorphism, International Dairy Federation, Brussels, Belgium (1997), pp. 372–394
B.L. Harris. Selection to increase β-lactoglobulin B variant in New Zealand dairy cattle. J.P. Hill, M. Boland (Eds.), Milk Protein Polymorphism, International Dairy Federation, Brussels, Belgium (1997), pp. 467–473
P. Havea, H. Singh, L.K. Creamer. Characterization of heat-induced aggregates of β-lactoglobulin, α-lactalbumin, and bovine serum albumin in a whey protein concentrate environment. J. Dairy Res., 68 (2001), pp. 483–497
D.R. Flower, A.C.T. North, C.E. Sansom The lipocalin protein family—structural and sequence overview. Biochim. Biophys. Acta, 1482 (2000), pp. 9–24
A.C. Ouwehand, S.J. Salminen, M. Skurnik, P.L. Conway Inhibition of pathogen adhesion by beta-lactoglobulin. Int. Dairy J., 7 (1997), pp. 685–692
J.M.A. Tilley. The chemical and physical properties of bovine β-lactoglobulin Dairy Sci. Abstr., 22 (1960), pp. 111–125
R.L.J. Lyster. Reviews of the progress of dairy science. Section C. Chemistry of milk proteins. J. Dairy Res., 39 (1972), pp. 279–318
J.E. Kinsella, D.M. Whitehead. Proteins in whey: Chemical, physical and functional properties. Adv. Food Nutr. Res., 33 (1989), pp. 343–438
S.G. Hambling, A.S. McAlpine, L. Sawyer. β-Lactoglobulin. P.F. Fox (Ed.), Advanced Dairy Chemistry-I: Proteins, Elsevier, London, UK (1992), pp. 140–191
L. Sawyer. β-Lactoglobulin. P.F. Fox, P. McSweeney (Eds.), Advanced Dairy Chemistry I (3rd ed.), Kluwer, Amsterdam, The Netherlands (2003), pp. 319–386