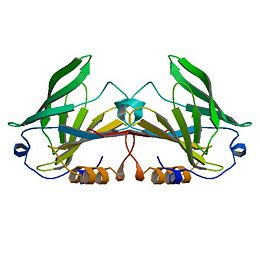
β-Lactoglobulin is a small protein, soluble in dilute salt solution as befits a globulin, with 162 amino acid residues (Mr ∼18,400) that fold up into an 8-stranded, antiparallel β-barrel with a 3-turn α-helix on the outer surface and a ninth β-strand flanking the first strand (see Figure 1). It is this strand that forms a significant part of the dimer interface in the bovine and ovine proteins but, while still present in porcine β-LG, is not involved in the formation of the dimer that forms at low pH. The genetic variants referred to above in the ruminant species result in relatively minor amino acid differences, but the processing properties appear to be significantly affected even by these small changes, such that consideration has been given to removing the less favorable variants by selective breeding (Hill et al., 1997; Harris, 1997). The so-called calyx, or β-barrel, is conical and is made of β-strands A-D forming one sheet, and strands E-H forming a second. Strand A bends through a right angle such that the C-terminal end forms an antiparallel strand with H; strands D and E also form a less significant interaction completely closing the calyx. It is this central cavity, the calyx, that provides the ligand-binding site. On the outer surface of the β-barrel, between strands G and H, is the 3-turn α-helix. The loops that connect the β-strands at the closed end of the calyx, BC, DE, and FG are generally quite short, whereas those at the open end are significantly longer and more flexible.
In particular, the EF loop acts as a gate over the binding site. At low pH, it is in the “closed” position, and binding is inhibited or impossible, whereas at high pH it is open, allowing ligands to penetrate into the hydrophobic binding site. The “latch” for this gate is Glu89, the residue implicated in the Tanford transition observed, although not identified, as having an abnormally high pKa (Tanford et al., 1959; Brownlow et al., 1997; Qin et al., 1998a).
The free Cys121, with its reactive thiol group, has a pH-dependent activity that parallels that of the Tanford transition, and its involvement in the denaturation and aggregation behavior is now firmly established (Havea et al., 2001). The residue is situated on the outer surface of the β-barrel on strand H, under the α-helix, and quite some distance from the EF loop. The effect of this is to mask its accessibility to solvent, particularly at low pH. Reaction with heavy metals like Hg2+ or Cd2+ does occur at most pH values and leads to a dissociation of the dimer. Indeed, reaction of the Cys with almost any thiol reagent appears to enhance dissociation into monomers (McKenzie and Sawyer, 1967; Hambling et al., 1992).
The CD and GH loops in many of the crystallographic analyses are indistinct, and the similarly indistinct nature of the extended AB loop led to the original misthreading of the amino acid sequence into the first electron density map (Papiz et al., 1986; Brownlow et al., 1997). β-Strand I is on the opposite side of strand A from strand H and forms an antiparallel interaction with the same strand of the second monomer. The other dimer-stabilizing interaction comes from the AB loop, where there is an ion pair from Asp33 of one subunit to Arg40 of the other. It is interesting to note that although strand I also exists in the porcine and equine β-LG structures, albeit with a slightly modified sequence, as do the Asp and Arg residues, no similar dimerization occurs at physiological pH. Indeed, site-directed mutagenesis designed to disrupt one or the other of these interactions, or better, both, produces a monomeric bovine protein (Sakurai and Goto, 2002; Wilson and Holt, to be published). There is no direct interaction between the α-helixes, which are antiparallel on the outer surface of the molecule with the polypeptide chains, some 12 Å apart. The closest approach of any atoms of the helix in the A subunit from the triclinic lattice X structure to the helix in the B subunit is 5.8Å, and this involves the flexible side chains of Asp137A and Arg148B and vice versa.
The structure of β-LG has also been determined by NMR techniques by several groups (Belloque and Smith, 1998; Fogolari et al., 1998; Kuwata et al., 1998; Uhrinova et al., 2000) and includes the equine structure (Kobayashi et al., 2000). The bovine protein is monomeric at pH values below 3, and the structure has been determined at this pH. Although there is no matching crystal structure at this pH where flat hexagonal plates of poor diffraction quality can be grown, the structure of the core of the protein is well conserved. However, significant differences do exist, and these have been critically reviewed (Jameson et al., 2002); it is not surprising that it is the external loops that contain these major differences.
No definite physiological function has been ascribed to β-LG, although several suggestions have been made, the more compelling of which favor a role in molecular transport or, possibly, as some form of modulator (Kontopidis et al., 2002). Most suggestions concerning the function, understandably, have concentrated on either the lactating cell or more usually the neonate, and a transporter role seems reasonable, since many lipocalins are transporters. β-Lactoglobulin binds both fatty acids and retinol, and the structure is similar to the known transporter, plasma RBP. The nature of the ligand transported, apart from being generally hydrophobic, is not clear, however. Fatty acids, rather than retinal, are found as endogenous ligands in milk, but not all species have a β-LG that binds fatty acids (Perez et al., 1989, 1993), and it seems improbable that the true function will vary from species to species. Similarly, retinol is significantly more soluble in the fat phase of milk and thus will probably be transferred from mother to offspring by that route. A signaling or activity-modulator role appears to be less likely, not just because of the paucity of similar roles reported for other lipocalins (Flower et al., 2000), but also because the data supporting these various activities appear rather circumstantial. Further, for such an important role, one might expect the presence of β-LG in the milks of all species, not just some. What does not appear to have been considered in detail until recently (Kontopidis et al., 2002) is that the function is directly related to maternal physiology.
When the species variation of β-LG sequences is examined along with that of the other lipocalins, what emerges is a family tree like that shown in Figure 4. The RBP are clearly distinct from the lactoglobulins, but there is one protein present in the endometrium, now called glycodelin (formerly PP14, inter alia), that is the closest relative to the β-LG-II gene product (Halttunen et al., 2000; Seppälä, 2002). There are also reports of RBP expressed in the endometrium of cow (Thomas et al., 1992; MacKenzie et al., 1997). Notice also that there is ruminant pseudo-gene identified in both cow and goat that is also most nearly related to the sequence of β-LG-II. Might it be that the protein glycodelin reflects the true, original function of β-LG as a protein involved in some aspect of fetal development in all mammals? In many species, the gene has undergone a gene duplication event and is expressed during lactation for nutritional purposes. More recently, some species, like rodents, lagomorphs, and man, have lost the function of one of these genes through formation of a pseudo-gene, resulting in the gene ceasing to be expressed. The biological properties of the milk protein, such as ligand binding and inhibiting harmful bacterial adhesion in the intestine (Ouwehand et al., 1997) that have been identified, are clearly useful to each species but are subsidiary to those of neonatal nutrition. On the other hand, it is known that low levels of β-LG are expressed in the cow throughout the dry period in a manner that is distinct from α-LA and the caseins (Aslam et al., 1994). Whether this is indicative of another more physiological role for β-LG in the changes that occur to the mammary gland during this period remains unclear at present.