User:Erin May/Sandbox 1
From Proteopedia
(→Dimer Form) |
(→Dimer Form) |
||
| Line 77: | Line 77: | ||
More Van der Waals and electrostatic forces occur between the <scene name='User:Erin_May/Sandbox_1/Switch-helix_1_interactions/1'>switch region and helix 1</scene> of the same molecule. These interactions would not be able to occur in the monomer. | More Van der Waals and electrostatic forces occur between the <scene name='User:Erin_May/Sandbox_1/Switch-helix_1_interactions/1'>switch region and helix 1</scene> of the same molecule. These interactions would not be able to occur in the monomer. | ||
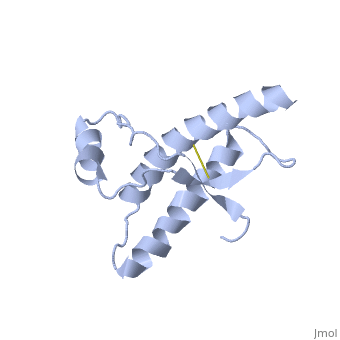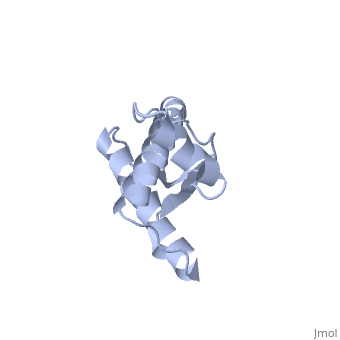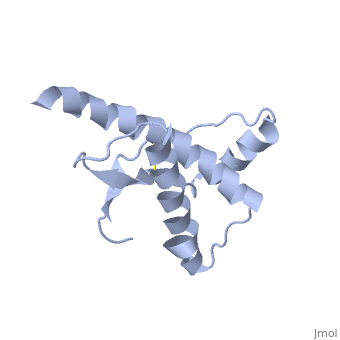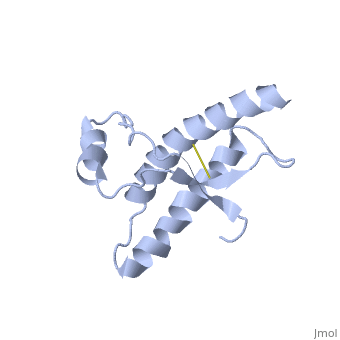
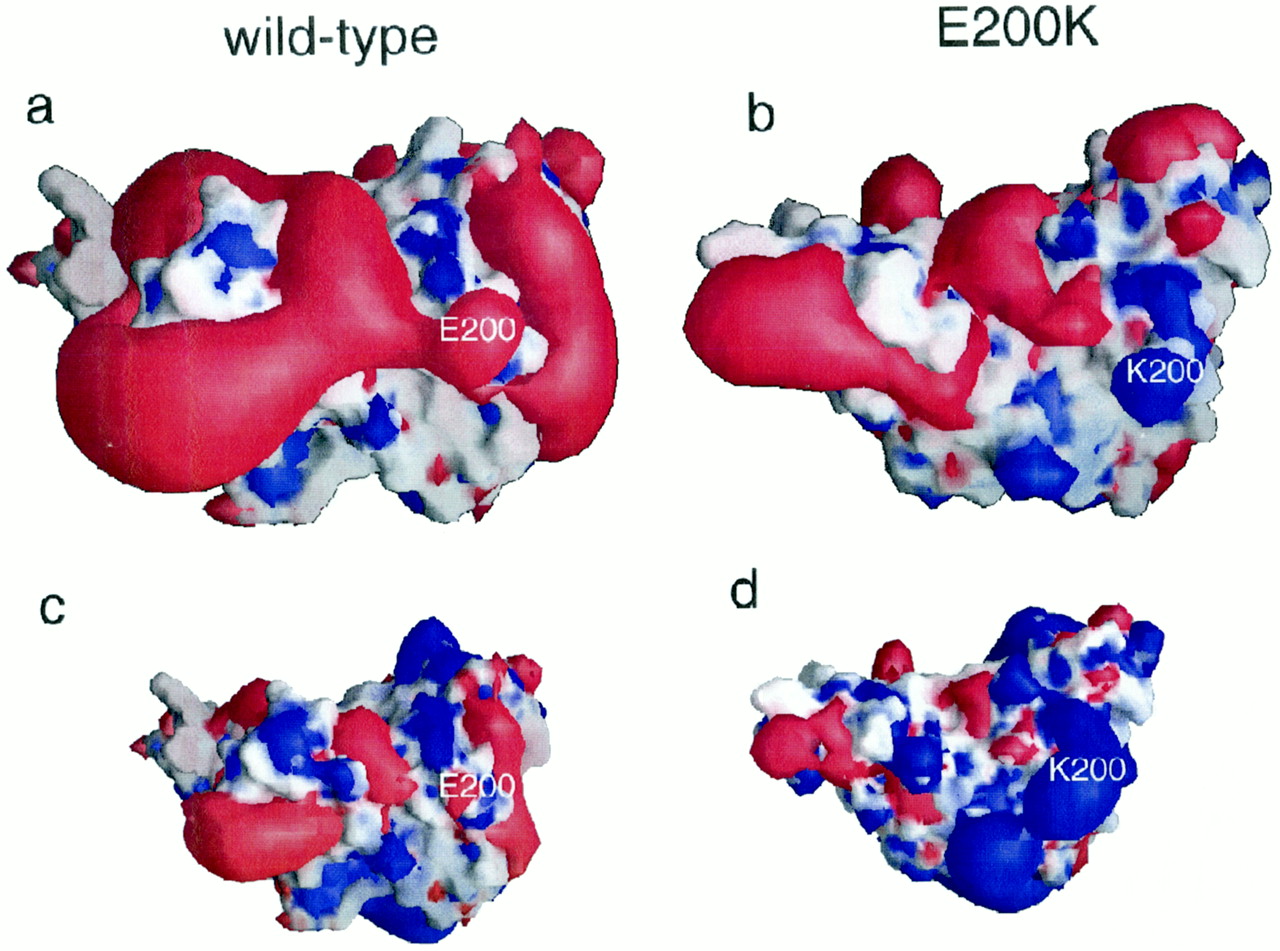
| - | + | <scene name='User:Erin_May/Sandbox_1/Interface_hydrogen_bonding/1'>Dimer interface hydrogen bonding</scene> between the dimers exist at Thr188 O−Gly195 N, Thr190 O−Lys194 N and Thr192 O−Thr192 N | |
<scene name='User:Erin_May/Sandbox_1/Hydrogen_bond_asp_202/1'>Hydrogen bonding</scene> between Asp 202 and Thr 199 stabilize the dimeric structure. | <scene name='User:Erin_May/Sandbox_1/Hydrogen_bond_asp_202/1'>Hydrogen bonding</scene> between Asp 202 and Thr 199 stabilize the dimeric structure. | ||
| - | <scene name='User:Erin_May/Sandbox_1/Hydrogen_bonding/1'>Arg 220 and Ser 132</scene> form a hydrogen bond | + | <scene name='User:Erin_May/Sandbox_1/Hydrogen_bonding/1'>Arg 220 and Ser 132</scene> form a hydrogen bond located at the end of helix 3 and near the switch region of the other chain, which allow the inter-chain interactions to be specific. |
Due to the importance of initial dimerization in the formation of prion aggregates, the step of dimerization presents a known step for treatments to target.<ref name="Knaus">PMID:11524679</ref> | Due to the importance of initial dimerization in the formation of prion aggregates, the step of dimerization presents a known step for treatments to target.<ref name="Knaus">PMID:11524679</ref> | ||
Revision as of 23:44, 27 November 2012
Contents |
Prions as a disease causing agent
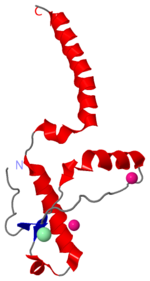
Prions are infectious or genetically coded misfolded proteins which act as templates upon which properly folded prion protein monomers can aggregate. Prions contain no nucleic acid such as other infectoius molecules or organisms. Human Prion Protein or Major Prion protein, exists as a normal constituent of human cells, found mostly in the brain[2] and is called PrPC.[3] PrPC is composed of mostly helix whereas the infectious form, PrPSc (also known as "scrapie" form), is composed of high percentage beta sheets.[3]
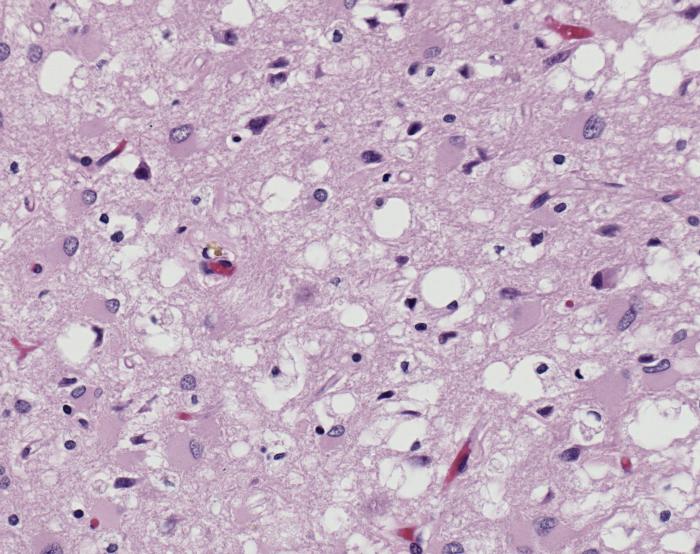
The diseases prions confer are neurodegenerative disorders which result from the large scale aggregation of these proteins. This "bubbles" of protein aggregates appear clear on a pictomicrograph and resemble a sponge. Bovine Spongiform Encephalopathy(BSE), or Mad Cow Disease, is a form of Transmissible Spongiform Encephalopathy caused by ingesting bovine prions. The first known cases of BSE occurred in the 1970's and have garnered a lot of media attention. Recently, feed bans in the United States and Canada have been adopted by the government in an attempt to stop the spread of BSE between cows. This bans the use of potential materials which would contain prion proteins, whether misfolded or wild-type. [4] For more information about the infections related to prions see Transmissible spongiform encephalopathy at Wikipedia.
Unfolding Mechanism
Currently, the mechanism by which a template prion unfolds a the helices of a properly folded prion protein is unknown. Specific residues have been shown to either confer resistance or lend themselves to this unfolding.
PrPC natural monomer
| |||||||||||
PrPSc
| |||||||||||
Dimer Form
| |||||||||||
Reference List
- ↑ Image of Creutzfeldt-Jakob positive brain tissue was obtained from The CDC's Public Health Image Library.
- ↑ Centers for Disease Control and Prevention: Prions. http://www.cdc.gov/ncidod/dvrd/prions/
- ↑ 3.0 3.1 Prusiner SB. Prions. Proc Natl Acad Sci U S A. 1998 Nov 10;95(23):13363-83. PMID:9811807
- ↑ Centers for Disease Control and Prevention: Bovine Spongiform Encephalopathy. http://www.cdc.gov/ncidod/dvrd/bse/index.htm
- ↑ 5.0 5.1 5.2 5.3 Lee S, Antony L, Hartmann R, Knaus KJ, Surewicz K, Surewicz WK, Yee VC. Conformational diversity in prion protein variants influences intermolecular beta-sheet formation. EMBO J. 2010 Jan 6;29(1):251-62. Epub 2009 Nov 19. PMID:19927125 doi:10.1038/emboj.2009.333
- ↑ 6.0 6.1 6.2 6.3 6.4 Knaus KJ, Morillas M, Swietnicki W, Malone M, Surewicz WK, Yee VC. Crystal structure of the human prion protein reveals a mechanism for oligomerization. Nat Struct Biol. 2001 Sep;8(9):770-4. PMID:11524679 doi:10.1038/nsb0901-770
- ↑ 7.0 7.1 7.2 7.3 Zhang Y, Swietnicki W, Zagorski MG, Surewicz WK, Sonnichsen FD. Solution structure of the E200K variant of human prion protein. Implications for the mechanism of pathogenesis in familial prion diseases. J Biol Chem. 2000 Oct 27;275(43):33650-4. PMID:10954699 doi:10.1074/jbc.C000483200