Adrenergic receptor
From Proteopedia
m |
m (→β-2 adrenergic receptor) |
||
| Line 24: | Line 24: | ||
===β-2 adrenergic receptor=== | ===β-2 adrenergic receptor=== | ||
| + | <table width=309' align='right' cellpadding='0'><tr><td rowspan='2'> </td><td bgcolor='#eeeeee'>[[Image:7tm labeled.png|right|300px]]</td></tr><tr><td bgcolor='#eeeeee'><center> | ||
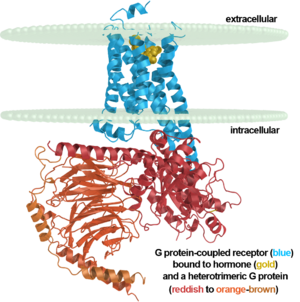
| + | β2 adrenergic receptor binding a hormone analog<br/> and complexed to a heterotrimeric G protein ([[3sn6]]) | ||
| + | </center></td></tr></table> | ||
{{Template:GPCR3sn6}} | {{Template:GPCR3sn6}} | ||
See also [[Beta-2 Adrenergic Receptor]]<br /> | See also [[Beta-2 Adrenergic Receptor]]<br /> | ||
Revision as of 04:17, 8 December 2012
| |||||||||
| β-2 Adrenergic Receptor, 2rh1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | , , , , , , , | ||||||||
| Gene: | ADRB2, ADRB2R, B2AR / E (Enterobacteria phage T4) | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
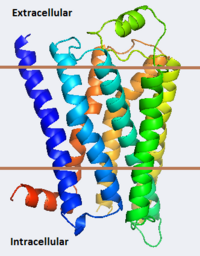
The adrenergic receptors are metabolic G protein-coupled receptors. They are the targets of catecholamines. The binding of an agonist to them causes a sympathetic response. The α-2 adrenergic receptor (A2AR) inhibits insulin or glucagons release. The β-1 adrenergic receptor (B1AR) increases cardiac output and secretion of rennin and ghrelin. The β-2 adrenergic receptor (B2AR) triggers many relaxation reactions. The images at the left and at the right correspond to one representative Adrenergic receptor, i.e. the crystal structure of human β-2 adrenergic Receptor (2rh1). See also Beta-2 Adrenergic Receptor and G protein-coupled receptor. (morph was taken from Gallery of Morphs of the Yale Morph Server).
Contents |
3D Structures of Adrenergic receptor
Update November 2011
α-2 adrenergic receptor
3kj6, 2r4r, 2r4s – hA2AR + FAB heavy+light chains
1ho9, 1hod – hA2AR peptide (mutant) – NMR
1hof, 1hll - hA2AR peptide – NMR
β-1 adrenergic receptor
2y01 – tB1AR fragment (mutant) – turkey
1dep – tB1AR peptide - NMR
2y00, 2y02, 2y03, 2y04, 2vt4 - tB1AR fragment (mutant) + agonist
2ycw, 2ycx, 2ycz, 2ycy - tB1AR fragment (mutant) + antagonist
β-2 adrenergic receptor
β2 adrenergic receptor binding a hormone analog |
| |||||||||||
| |||||||||||
See also Beta-2 Adrenergic Receptor
3pds - hB2AR/T4 lysozyme - human
3p0g – hB2AR/T4 lysozyme + cameloid antibody fragment
2r4s,2r4r - hB2AR + Fab5 complex
2rh1 - hB2AR/T4 lysozyme (mutant)
3d4s - hB2AR/T4 lysozyme (mutant) + cholesterol
3ny8, 3ny9, 3nya – hB2AR + agonist
3sn6 - human β-2 adrenergic receptor + cameloid antibody fragment bound by guanine nucleotide-binding protein G. This is the first structure of an activated GPCR in a complex with its G protein.
See also The Madison West High School 2008 SMART Team's Page on the β-2 adrenergic receptor
Nobel Prize Related to the Structures
Robert J. Lefkowitz and Brian K. Kobilka share the 2012 Nobel Prize in Chemistry for work on GPCRs that includes solving the first structures of a ligand-activated GPCR (2r4r, 2r4s, & 2rh1 in 2007)[1][2][3] and the first activated GPCR in complex with its G protein (3sn6 in 2011)[4][5][6][7]. A detailed description of the laureates' body of work on this class of receptors with images is here.
References and Notes
See Also
- G protein-coupled receptor
- Beta-2 Adrenergic Receptor topic page
- Nobel Prizes for 3D Molecular Structure
- Highest impact structures of all time
- G proteins
- Rhodopsin
- GTP-binding protein
- Pharmaceutical Drugs
- Membrane proteins
- Hormone
External Resources
- Robert J. Lefkowitz and Brian K. Kobilka share the 2012 Nobel Prize in Chemistry for work on GPCRs that includes solving the first structures of a ligand-activated GPCR (2007) and the first activated GPCR in complex with its G protein (2011). A detailed description of the laureates' body of work on this class of receptors with images is here.
- The April 2008 RCSB PDB Molecule of the Month feature on Adrenergic Receptors by David S. Goodsell is 10.2210/rcsb_pdb/mom_2008_4.
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Wayne Decatur, Karsten Theis, Alexander Berchansky