Amyloid precursor protein
From Proteopedia
| Line 1: | Line 1: | ||
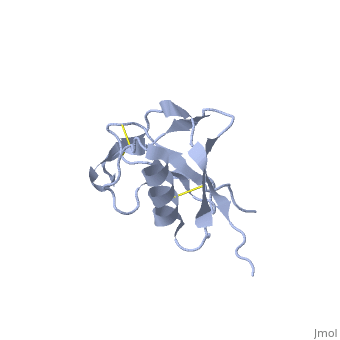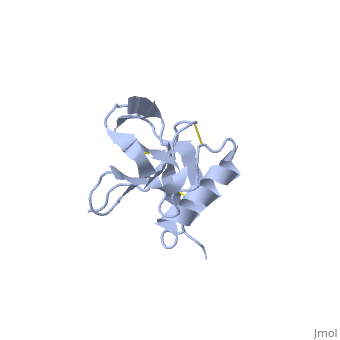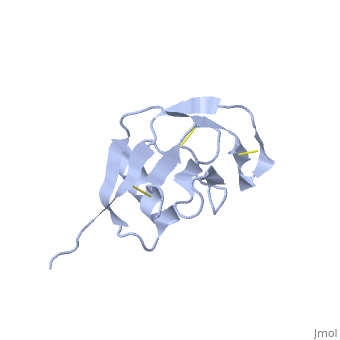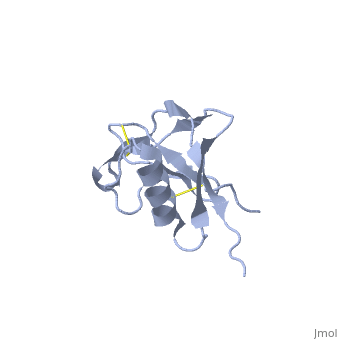
{{STRUCTURE_1mwp| PDB=1mwp | SIZE=400| SCENE= |right| CAPTION=Human amyloid precursor protein heparin-binding domain [[1mwp]] }} | {{STRUCTURE_1mwp| PDB=1mwp | SIZE=400| SCENE= |right| CAPTION=Human amyloid precursor protein heparin-binding domain [[1mwp]] }} | ||
| - | '''Amyloid precursor protein''' (APP) is thought to regulate transcription. For detailed discussion see [[Human APP]] | + | '''Amyloid precursor protein''' (APP) is thought to regulate transcription. APP is cleaved by β-secretase and the resulting N terminal peptide which is ca. 40 amino acid long is called hAPP β-peptide. The aggregation of the hAPP β-peptide is the cause of Alzheimer’s Disease. For detailed discussion see<br /> |
| + | * [[Human APP]]<br /> | ||
| + | * [[Human APP Intracellular Domain Complex with Fe65-PTB2]]<br /> | ||
| + | * [[Amyloid beta]]. | ||
__NOTOC__ | __NOTOC__ | ||
| Line 11: | Line 14: | ||
[[3nyj]], [[3nyl]], [[3umh]], [[3umi]], [[3umk]] – hAPP E2 domain – human<BR /> | [[3nyj]], [[3nyl]], [[3umh]], [[3umi]], [[3umk]] – hAPP E2 domain – human<BR /> | ||
[[3ktm]] – hAPP residues 18-190<BR /> | [[3ktm]] – hAPP residues 18-190<BR /> | ||
| - | [[1z0q]], [[2beg]] – hAPP β-peptide – NMR<BR /> | ||
[[1ze7]], [[2bp4]] – hAPP zinc-binding domain – NMR<BR /> | [[1ze7]], [[2bp4]] – hAPP zinc-binding domain – NMR<BR /> | ||
[[2fjz]], [[2fma]] - hAPP residues 133-189<BR /> | [[2fjz]], [[2fma]] - hAPP residues 133-189<BR /> | ||
| Line 19: | Line 21: | ||
[[1qyt]], [[1qwp]], [[1qxc]] - hAPP residues 25-35 – NMR<BR /> | [[1qyt]], [[1qwp]], [[1qxc]] - hAPP residues 25-35 – NMR<BR /> | ||
[[1mwp]] - hAPP heparin-binding domain<br /> | [[1mwp]] - hAPP heparin-binding domain<br /> | ||
| + | |||
| + | ===hAPP β-peptide=== | ||
| + | |||
| + | [[1z0q]], [[2beg]] – hAPP β-peptide residues 1-42 – NMR<br /> | ||
| + | [[1amb]], [[1amc]] – hAPP β-peptide residues 1-28 – NMR<br /> | ||
| + | [[1qcm]], [[1qwp]], [[1qxc]], [[1qyt]] – hAPP β-peptide residues 25-35 – NMR<br /> | ||
| + | [[1ba4]], [[1ba6]], [[2lnq]] – hAPP β-peptide residues 1-40 – NMR<br /> | ||
| + | [[1bjb]], [[1bjc]] – hAPP β-peptide residues 1-28 (mutant) – NMR<br /> | ||
| + | [[1nmj]] – APP β-peptide residues 1-28 – rat – NMR<br /> | ||
| + | [[3bae]] – hAPP β-peptide residues 1-28 + antibody<br /> | ||
| + | [[3bkj]] – hAPP β-peptide residues 1-16 + antibody<br /> | ||
| + | [[2roz]] – hAPP β-peptide residues 1-32 + amyloid β A4 precursor protein - NMR<br /> | ||
| + | [[2wk3]] – hAPP β-peptide residues 1-42 + insulin-degrading enzyme<br /> | ||
===Amyloid precursor protein binary complex=== | ===Amyloid precursor protein binary complex=== | ||
Revision as of 09:23, 9 April 2013
| |||||||||
| Human amyloid precursor protein heparin-binding domain 1mwp | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
Amyloid precursor protein (APP) is thought to regulate transcription. APP is cleaved by β-secretase and the resulting N terminal peptide which is ca. 40 amino acid long is called hAPP β-peptide. The aggregation of the hAPP β-peptide is the cause of Alzheimer’s Disease. For detailed discussion see
3D structures of amyloid precursor protein
Updated on 09-April-2013
3nyj, 3nyl, 3umh, 3umi, 3umk – hAPP E2 domain – human
3ktm – hAPP residues 18-190
1ze7, 2bp4 – hAPP zinc-binding domain – NMR
2fjz, 2fma - hAPP residues 133-189
1owt - hAPP residues 133-189 - NMR
1rw6 - hAPP residues 346-551
1tkn - hAPP residues 460-569 - NMR
1qyt, 1qwp, 1qxc - hAPP residues 25-35 – NMR
1mwp - hAPP heparin-binding domain
hAPP β-peptide
1z0q, 2beg – hAPP β-peptide residues 1-42 – NMR
1amb, 1amc – hAPP β-peptide residues 1-28 – NMR
1qcm, 1qwp, 1qxc, 1qyt – hAPP β-peptide residues 25-35 – NMR
1ba4, 1ba6, 2lnq – hAPP β-peptide residues 1-40 – NMR
1bjb, 1bjc – hAPP β-peptide residues 1-28 (mutant) – NMR
1nmj – APP β-peptide residues 1-28 – rat – NMR
3bae – hAPP β-peptide residues 1-28 + antibody
3bkj – hAPP β-peptide residues 1-16 + antibody
2roz – hAPP β-peptide residues 1-32 + amyloid β A4 precursor protein - NMR
2wk3 – hAPP β-peptide residues 1-42 + insulin-degrading enzyme
Amyloid precursor protein binary complex
1ze9 - hAPP zinc-binding domain + Zn – NMR
2fk1, 2fk2, 2fk3, 2fkl - hAPP residues 133-189 + Cu
3jti, 3gci – hAPP peptide + phospholipase A2
3l81 - hAPP peptide + AP-4 complex subunit μ-1
3ifl, 3ifn, 3ifo, 3ifp, 2r0w - hAPP peptide + antibody
2wk3 - hAPP residues 1-42 + insulin degrading enzyme
3dxc - hAPP residues 739-770 + Fe65-PTB2
3dxd, 3dxe - hAPP residues 739-770 (mutant) + Fe65-PTB2
2roz - hAPP peptide + Fe65 C terminal
2otk - hAPP residues 672-711 + ZAB3 affibody dimer - NMR
3l3t - hAPP residues 211-267 + mesotrypsin
3l33 - hAPP residues 290-341 + trypsin-3 (mutant)