Sandbox Reserved 592
From Proteopedia
| Line 41: | Line 41: | ||
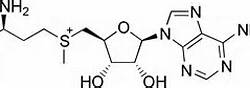
There are two types of histone methyltransferases: lysine specific and argine specific; each is a residue which the enzyme transfers a methyl group to. Within the lysine specific, there are further two main types: SET (Su(var)3-9, Enhancer of Zeste, Trithorax) and non-SET domains. These domains are an essential part of the enzyme because the main catalytic occurs in SET domain of the methyltransferase. SUV39h1 is considered to have a lysine specific SET domain which catalyzes the methyltransferase. In the nucleosomes, there are four different proteins; each consists of two copies which make the entire nucleosome. The mechanism by which SUV39H1 methylates the histone proteins is also known. A nearby tyrosine residue in the histone protein creates a strong nucleophile by deprotonating a nearby lysine residue in the same histone protein. The lysine residue is a very strong nucleophile which attacks the sulfur atom of the cofactor S-Adensyl methoinine (SAM) and extracts a methyl group from the cofactor. Subsequently, the attack methylates the histone protein (Shown in Figure 4). The cofactor is very important because it is the source of the methyl group. | There are two types of histone methyltransferases: lysine specific and argine specific; each is a residue which the enzyme transfers a methyl group to. Within the lysine specific, there are further two main types: SET (Su(var)3-9, Enhancer of Zeste, Trithorax) and non-SET domains. These domains are an essential part of the enzyme because the main catalytic occurs in SET domain of the methyltransferase. SUV39h1 is considered to have a lysine specific SET domain which catalyzes the methyltransferase. In the nucleosomes, there are four different proteins; each consists of two copies which make the entire nucleosome. The mechanism by which SUV39H1 methylates the histone proteins is also known. A nearby tyrosine residue in the histone protein creates a strong nucleophile by deprotonating a nearby lysine residue in the same histone protein. The lysine residue is a very strong nucleophile which attacks the sulfur atom of the cofactor S-Adensyl methoinine (SAM) and extracts a methyl group from the cofactor. Subsequently, the attack methylates the histone protein (Shown in Figure 4). The cofactor is very important because it is the source of the methyl group. | ||
=== Chromodomain function === | === Chromodomain function === | ||
| - | The chromodomain structure of the enzyme (residues) is not involve in the catalytic function of the enzyme, but is very important to the binding to the molecule. Mutations which remove the chromodomain area of the enzyme result in the inhibition of the enzyme. The inhibition of the chromodomain prevents the methylating action of the enzyme's catalytic domain. Although the chromodomain is not directly involved in the methylation of the histone proteins, it plays an important role in the binding of the enzyme to histone proteins as well as other proteins. Fluorescence Polarization Assays have shown the chromodomain has a high affinity for the histone protein H3K9me1 and H3K9me2. SUV39H1 residues that did not contain the aromatic F34 cage did not bind to the histone protein. This shows the F34 is a conserved residue that is essential for the enzyme binding to the histone protein. Moreover, a mutation which results in the loss of the aromatic F34 cage group will inhibit the binding effect of the chromodomain enzymes. SUV39H1 does not methylate the histone protein alone; it is aided by other proteins. Studies have shown HP1 and H3 are essential proteins that combine with SUV39H1 in protein complex, which methylate the histone protein of a nucleosome.--- | + | The chromodomain structure of the enzyme (residues) is not involve in the catalytic function of the enzyme, but is very important to the binding to the molecule. Mutations which remove the chromodomain area of the enzyme result in the inhibition of the enzyme. The inhibition of the chromodomain prevents the methylating action of the enzyme's catalytic domain. Although the chromodomain is not directly involved in the methylation of the histone proteins, it plays an important role in the binding of the enzyme to histone proteins as well as other proteins. Fluorescence Polarization Assays have shown the chromodomain has a high affinity for the histone protein H3K9me1 and H3K9me2. SUV39H1 residues that did not contain the aromatic F34 cage did not bind to the histone protein. This shows the F34 is a conserved residue that is essential for the enzyme binding to the histone protein. Moreover, a mutation which results in the loss of the aromatic F34 cage group will inhibit the binding effect of the chromodomain enzymes. SUV39H1 does not methylate the histone protein alone; it is aided by other proteins. Studies have shown HP1 and H3 are essential proteins that combine with SUV39H1 in protein complex, which methylate the histone protein of a nucleosome. |
| + | |||
| + | |||
| + | {| align=left | ||
| + | |- | ||
| + | | | ||
| + | |||
| + | [[Image:picture ta.jpg| 300 px | thumb |Figure 3 showing the methylation of the histone protein and the subsequent formation of heterochromatin]] | ||
| + | |||
| + | |||
| + | |||
| + | ---- | ||
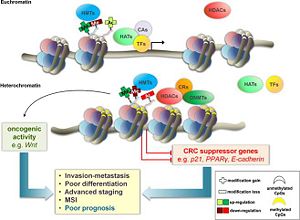
== Clinical relevence == | == Clinical relevence == | ||
Revision as of 19:16, 25 April 2013
==Your Heading Here (maybe something like 'Structure'-- PLEASE DO NOT DELETE THIS TEMPLATE -->
| This Sandbox is Reserved from Feb 1, 2013, through May 10, 2013 for use in the course "Biochemistry" taught by Irma Santoro at the Reinhardt University. This reservation includes Sandbox Reserved 591 through Sandbox Reserved 599. |
To get started:
More help: Help:Editing |
Contents |
Background
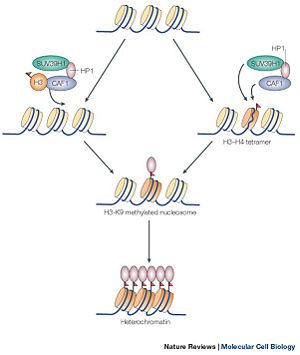
SUV39h1 is part of a class of methytransferase that deals with the methylation of histone proteins in nucleosomes. It is one of the very first histone methyltransferase to be discovered. The methylation of histone proteins is an essential part of an area of genetics which deals with the modification of histone proteins called epigenomics. Epigenomics is the study of modifications of nucleosomes, which either inhibit or express gene transcription without changing the underlining DNA sequence. These changes are allowed because of the amino acid tails which extend out and away from the histone proteins, exposing itself to methylation. Methylation of histone protein is one of many ways that regulates gene expression. The regulation of gene expression is dependent on the state of the gene. A gene cannot be transcribed when the promoter of the gene is wrapped around the nucleosome, forming a heterochromatin state; thus, the gene is inhibited from being transcribed. Conversely, Methylation of the histone protein causes the winding around the nucleosomes, transforming a euchromatin state into a heterochromatin state. Of course, SUV39H1 alone does not methylate the histone proteins. SUV39H1 interacts with other proteins in order to efficiently methylate a histone protein (shown in Figure 1). Abnormal expression of genes, which is the result of down regulation or mutation of SUV39H1, results in a variety of diseases.