Sandbox Reserved 690
From Proteopedia
| Line 14: | Line 14: | ||
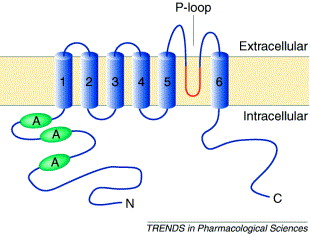
TRPV1 consists of 6 transmembrane domains. In the image of actual TRPV1 given, they are characterized by different colors.The "TRPV1" image also shows 3 distinct protein groups, A is shown in red, B is shown in green, and C is shown in blue. One can see, the <scene name='Sandbox_Reserved_690/Secondary_structure/1'>Secondary Structure</scene> of TRPV1 contains both beta sheets and alpha helices, with the alpha helices being the ones that cross the membrane.[[Image:Trpv1 pip2 bilayer.png]] | TRPV1 consists of 6 transmembrane domains. In the image of actual TRPV1 given, they are characterized by different colors.The "TRPV1" image also shows 3 distinct protein groups, A is shown in red, B is shown in green, and C is shown in blue. One can see, the <scene name='Sandbox_Reserved_690/Secondary_structure/1'>Secondary Structure</scene> of TRPV1 contains both beta sheets and alpha helices, with the alpha helices being the ones that cross the membrane.[[Image:Trpv1 pip2 bilayer.png]] | ||
| - | As TRPV1 is a transmembrane protein, it consists on both <scene name='Sandbox_Reserved_690/Polar_charged_residues/1'>hydrophobic</scene> and hydrophillic residues. The hydrophobic regions are shown in blue, and hydrophillic in red. Between the 5 and 6 domains of TRPV1 there consists a hydrophobic channel in which the cations flow through. | + | As TRPV1 is a transmembrane protein, it consists on both <scene name='Sandbox_Reserved_690/Polar_charged_residues/1'>hydrophobic</scene> and hydrophillic residues. The hydrophobic regions are shown in blue, and hydrophillic in red. Between the 5 and 6 domains of TRPV1 there consists a hydrophobic channel in which the cations flow through(Shown in the image below). |
[[Image:proteoTRPV1.jpg]] | [[Image:proteoTRPV1.jpg]] | ||
| Line 20: | Line 20: | ||
Hydrogen bonds did not work. so i didn't make an awkward non-working link. | Hydrogen bonds did not work. so i didn't make an awkward non-working link. | ||
| + | ==Interactions== | ||
| + | TRPV1 respondds to a variety of of agonists including capsaicin[http://en.wikipedia.org/wiki/Capsaicin], protons and heat. The sites of <scene name='Sandbox_Reserved_690/Interact_wit_ligand/1'>interaction</scene>, shown in lime green, occur on the C subunit (shown at the top) and the A subunit (bottom). The interractions of protons and capsaicin occur on the C subunit, and many of the regulation sites and heat become active on the A subunit. As this is not the real TRPV1 receptor, I am unable to show specific binding sites, however capsaicin binds to Arg 114, Tyr 511, Ser 512, Thr 550 and Glu 761 of TRPV1, all of which occur on the intracellular portion of the protein on the 3 and 5 domains. Protons interact with to Glu 600 and Glu 648, on the outer portion of the protein. Heat promotes its effects through interaction with the distal half of the C-terminus. | ||
| - | + | lime green | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
Revision as of 03:02, 3 May 2013
| This Sandbox is Reserved from 30/01/2013, through 30/12/2013 for use in the course "Biochemistry II" taught by Hannah Tims at the Messiah College. This reservation includes Sandbox Reserved 686 through Sandbox Reserved 700. |
To get started:
More help: Help:Editing |
| Please do NOT make changes to this Sandbox. Sandboxes 30-60 are reserved for use by Biochemistry 410 & 412 at Messiah College taught by Dr. Hannah Tims during Fall 2012 and Spring 2013. |
|
TRPV1
TRPV1[1] is also known as the capsaicin recpetor and VR1(vanilloid receptor 1). TRPV1 stands for transient receptor potential cation channel subfamily V member 1. TRPV1 is a protein responsible for detecting and regulating body temperature. It also is a nociceptor, meaning it also provides the sensations of heat and pain to the body. TRPV1 is found primarily in the dorsal root ganglia neurons of the body.
Please note that images are from an analog of TRPV1, which scientists then modify on computers to turn into TRPV1. As I do not have the software nor time to do so, I will be using the analog to discuss TRPV1 referencing it's active sites and such in the box while explaining the active sites of TRPV1. To reiterate, this is not TRPV1. We are just pretending it is.
Structure
TRPV1 consists of 6 transmembrane domains. In the image of actual TRPV1 given, they are characterized by different colors.The "TRPV1" image also shows 3 distinct protein groups, A is shown in red, B is shown in green, and C is shown in blue. One can see, the of TRPV1 contains both beta sheets and alpha helices, with the alpha helices being the ones that cross the membrane.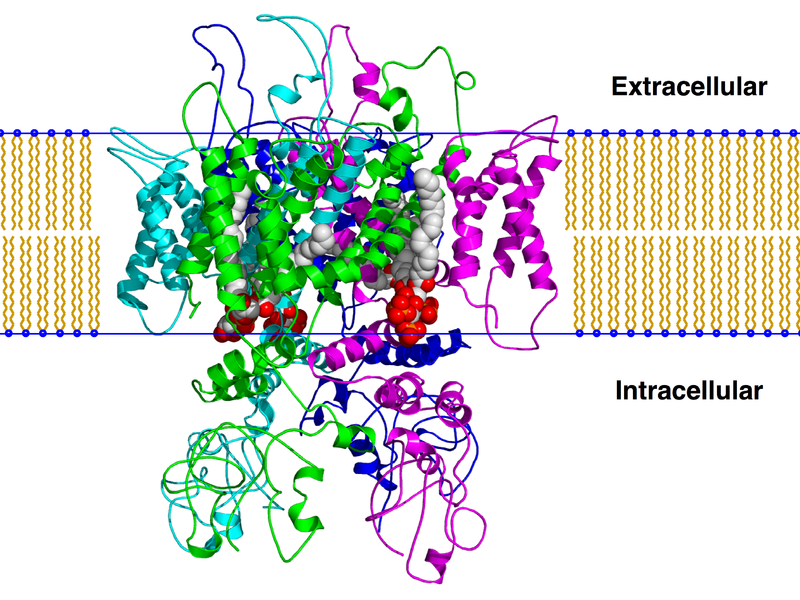
As TRPV1 is a transmembrane protein, it consists on both and hydrophillic residues. The hydrophobic regions are shown in blue, and hydrophillic in red. Between the 5 and 6 domains of TRPV1 there consists a hydrophobic channel in which the cations flow through(Shown in the image below).
Hydrogen bonds did not work. so i didn't make an awkward non-working link.
Interactions
TRPV1 respondds to a variety of of agonists including capsaicin[2], protons and heat. The sites of , shown in lime green, occur on the C subunit (shown at the top) and the A subunit (bottom). The interractions of protons and capsaicin occur on the C subunit, and many of the regulation sites and heat become active on the A subunit. As this is not the real TRPV1 receptor, I am unable to show specific binding sites, however capsaicin binds to Arg 114, Tyr 511, Ser 512, Thr 550 and Glu 761 of TRPV1, all of which occur on the intracellular portion of the protein on the 3 and 5 domains. Protons interact with to Glu 600 and Glu 648, on the outer portion of the protein. Heat promotes its effects through interaction with the distal half of the C-terminus.
lime green