SB2013 L04gr5
From Proteopedia
| Line 6: | Line 6: | ||
Within the varaible domain there are invariable regions that remain unchanged during antigenic varaition, and therefore may be targets of an immune response. IR6, the most conserved IR, has been found to be immnodominant. The antigencity of each of the 6 IRs have been studied using peptide-based enzyme linked immunosorbent essays, ELISA.(Liang et al. 1999). | Within the varaible domain there are invariable regions that remain unchanged during antigenic varaition, and therefore may be targets of an immune response. IR6, the most conserved IR, has been found to be immnodominant. The antigencity of each of the 6 IRs have been studied using peptide-based enzyme linked immunosorbent essays, ELISA.(Liang et al. 1999). | ||
| + | |||
| + | ==Structure== | ||
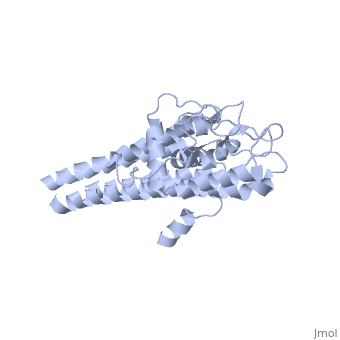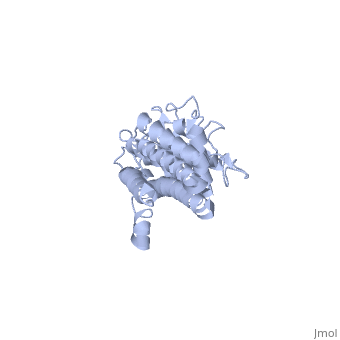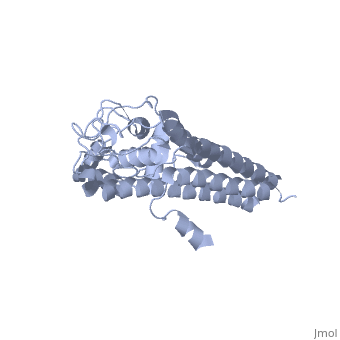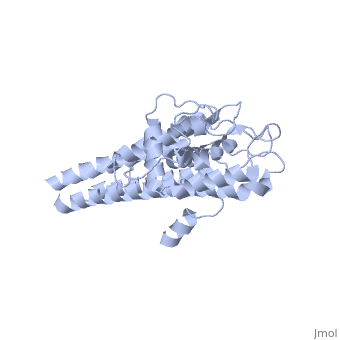
| + | The lipoprotein, VlsE, consists of two invariable domains at the amino and carboxyl termini and a variable domain. When referring to the primary structure of the protein, the variable domain is a cassette region located between the two termini. The variable domain can be further broken down into variable regions (VRs) and invariable regions (IRs) (Liang et al. 1999). When crystallized, VlsE forms a four molecule asymmetric unit (image of the unit) with each molecule having slight differences in their conformation. Although each molecule in the unit is slightly different, a single molecule of the protein consists of eleven α-helices and four short β-strands. Helices α1 (aa 306-341), α2 (aa 68-87), α3 (aa 114-139), and α11 (aa 306-341) all form the membrane proximal region of VlsE while helices α4 through α10 form the primary region of the membrane distal area of the protein. The four short β-strands each consist of 3 amino acids and can also be located in the membrane distal region. Covering the membrane distal part of VlsE are connecting loop regions, which lack any secondary structure and have different conformations in each of the molecules. (Eicken et al. 2002). The helices α3 through α10 form the invariable regions and are connected by the connecting loops which are classified as the variable regions. Although VlsE crystallizes into an asymmetrical unit, it appears primarily as monomeric in solution. Because the interface between VlsE molecules in the crystal structure buries approximately 13% of the accessible surface area of the monomers, Eicken et al.suggest that there is a possibility of VlsE existing as a dimer when in its natural state (possible image). | ||
| + | |||
Revision as of 02:33, 7 May 2013
VlsE
Lyme disease is the most pervasive tick-borne disease in Europe, the United States, and parts of Asia (Couette et al. 2009). It is a multistage infection caused by the spirochete Borrelia burgdorferi. Early symptoms include headaches, depression, rash, and fever. If Lyme disease is left untreated serious complications of the joints, heart and central nervous system can occur. Infected Ixodes ticks in their nymph stage transmit Lyme disease by attaching to humans and other mammals (Lab tutor). In most cases, the tick must be attached for 36-48 hours before the bacteria can be transmitted (CDC website).
VlsE, Vmp-like sequence Expressed protein, is a lipoprotein on the surface of Borrelia burgdorferi. VlsE contributes to the immune evasion and persistence of Lyme disease (Couette et al. 2009). VlsE contains invaraible and varaible domains. It was found that the varaible domain is highly immunogenic and the target of the immune response; however, through antigenic variation the lipoprotein is able to evade the host immune system (Liang et al. 1999).
Within the varaible domain there are invariable regions that remain unchanged during antigenic varaition, and therefore may be targets of an immune response. IR6, the most conserved IR, has been found to be immnodominant. The antigencity of each of the 6 IRs have been studied using peptide-based enzyme linked immunosorbent essays, ELISA.(Liang et al. 1999).
Structure
The lipoprotein, VlsE, consists of two invariable domains at the amino and carboxyl termini and a variable domain. When referring to the primary structure of the protein, the variable domain is a cassette region located between the two termini. The variable domain can be further broken down into variable regions (VRs) and invariable regions (IRs) (Liang et al. 1999). When crystallized, VlsE forms a four molecule asymmetric unit (image of the unit) with each molecule having slight differences in their conformation. Although each molecule in the unit is slightly different, a single molecule of the protein consists of eleven α-helices and four short β-strands. Helices α1 (aa 306-341), α2 (aa 68-87), α3 (aa 114-139), and α11 (aa 306-341) all form the membrane proximal region of VlsE while helices α4 through α10 form the primary region of the membrane distal area of the protein. The four short β-strands each consist of 3 amino acids and can also be located in the membrane distal region. Covering the membrane distal part of VlsE are connecting loop regions, which lack any secondary structure and have different conformations in each of the molecules. (Eicken et al. 2002). The helices α3 through α10 form the invariable regions and are connected by the connecting loops which are classified as the variable regions. Although VlsE crystallizes into an asymmetrical unit, it appears primarily as monomeric in solution. Because the interface between VlsE molecules in the crystal structure buries approximately 13% of the accessible surface area of the monomers, Eicken et al.suggest that there is a possibility of VlsE existing as a dimer when in its natural state (possible image).
|
|
Proteopedia Page Contributors and Editors (what is this?)
Emma Brower, Julia Joseph, Alexandra DePastene, Olivia Rodrigues, Alexander Berchansky, Michal Harel