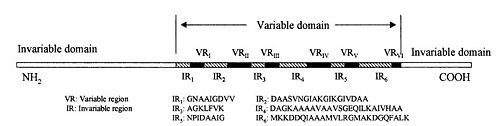
VlsE
Lyme disease is the most pervasive tick-borne disease in Europe, the United States,and parts of Asia.[1] It is a multistage infection caused by the spirochete Borrelia burgdorferi. Early symptoms include headaches, depression, rash, and fever. If Lyme disease is left untreated, serious complications of the joints, heart and central nervous system can occur. Infected Ixodes ticks in their nymph stage transmit Lyme disease by attaching to humans and other mammals[2]. In most cases, the tick must be attached for 36-48 hours before the bacteria can be transmitted.[3]
Vmp-like sequence Expressed protein (VlsE), is a lipoprotein on the surface of Borrelia burgdorferi. VlsE contributes to the immune evasion and persistence of Lyme disease.[1] VlsE contains invariable and variable domains. It was found that the varaible domain is highly immunogenic and the target of the immune response; however, through antigenic variation the lipoprotein is able to evade the host immune system.[4]
Within the variable domain, there are invariable regions that remain unchanged during antigenic variation, and therefore may be targets of an immune response. IR6, the most conserved IR, has been found to be immunodominant. The antigencity of each of the 6 IRs has been studied using peptide-based enzyme linked immunosorbent essays, ELISA.[4]
Structure
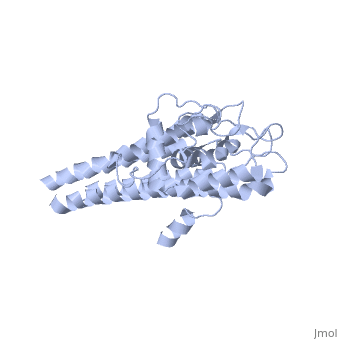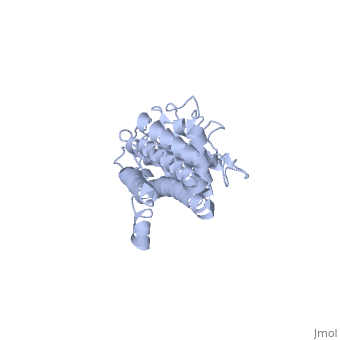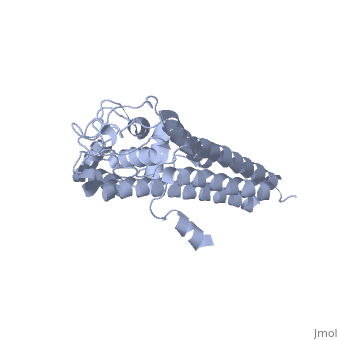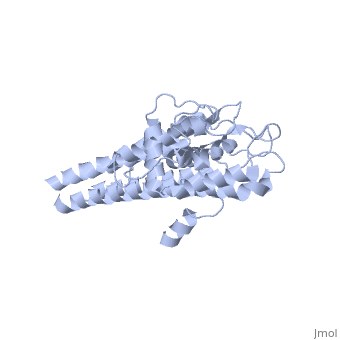
The lipoprotein, VlsE, consists of two invariable domains at the amino and carboxyl termini and a variable domain (Figure 1). When referring to the primary structure of the protein, the variable domain is a cassette region located between the two termini. The variable domain can be further broken down into variable regions () and invariable regions () [4].
When crystallized, VlsE forms a four molecule asymmetric unit with each molecule having slight differences in their conformation. Although each molecule in the unit is slightly different, a single molecule of the protein consists of eleven α-helices and four short β-strands. Helices α1 (aa 306-341), α2 (aa 68-87), α3 (aa 114-139), and α11 (aa 306-341) all form the of VlsE, while helices α4 (aa 161-176), α5 (aa 180-185), α6 (aa 195-201), α7 (aa 213-224), α8 (aa 228-239), α9 (aa 255-260) and α10 (aa 277-289) form the primary region of the of the protein. The four short β-strands each consist of 3 amino acids and can also be located in the membrane distal region [5].
Covering the membrane distal part of VlsE are connecting loop regions, which lack secondary structure and have different conformations in each of the molecules. Helices α3 through α10 form the invariable regions and are attached by the connecting loops that are classified as the variable regions. Although VlsE crystallizes into an asymmetrical unit, it appears primarily as monomeric in solution. Because the interface between VlsE molecules in the crystal structure buries approximately 13% of the accessible surface area of the monomers, research suggests that there is a possibility of VlsE existing as a dimer when in its natural state (Figure 2)[5].
Figure 1 shows the different domains of VlsE using a simplified 2-D representation of the lipoprotein.
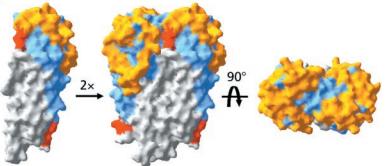
Figure 2 shows the possible dimerization of VlsE.
Antigenic Variation
Antigenic variation is the process by which an organism is able to evade its host’s immune system. The antigenic variation that occurs in VlsE uses a complex genetic conversion mechanism that is seen to be concentrated in the VRs. The complex genetic system for VlsE is located near the right telomere on a 28-kb linear plasmid (lp28-1) in strains of B. burgdorferi. This locus has been shown to be a crucial element for the persistence and virulence of Lyme disease [6]. The vls antigenic variation locus consists of a vls expression site (vlsE) and 15 silent vls cassettes that reside just upstream of the site. The vlsE cassette region, which is the variable domain and does not include the invariable amino or carboxyl termini, has approximately 92% DNA sequence identity with the silent vls cassettes. However, the silent vls cassettes lack promoter sequences and are therefore not expressed. Throughout the course of infection, the sequences for the flanking termini and the silent vls cassettes are conserved while the vlsE sequence is recombined. This suggests that the genetic variation mechanism occurs by copying segments of the 15 silent vls cassettes and completely replacing corresponding segments of vlsE sequences. The resulting differences are centralized in the highly variable regions of the vls cassettes. This allows for the constant evolution of the VR structures and evades the antibodies of the host immune system[7].
Variable Regions
The variable regions are an important component of the variable domain of VlsE. These regions have relatively high surface exposure, which accounts for approximately 37% of the total surface area of the crystallized protein monomer. The strategic location of the variable regions has been implicated in protecting the highly conserved invariable regions from antibody binding. These regions, which are almost completely buried within the membrane distal region, are surrounded by the protective loops of the variable regions.[5]
The variable regions’ location also supports the potential role that the protein plays in evading host immune responses through gene conversion.[7] These regions are hypothesized to expose highly variable epitopes, which would prevent the immune system from recognizing the antigen and subsequently prevent the onset of Lyme disease.[5] This feature starkly contrasts to that of the invariable regions, which does not undergo constant variation and whose genetic composition remains mostly conserved.[7]
Invariable Regions
Interspersed in the variable regions of the variable domain are six invariable regions (IR1-IR6). Although located on the membrane distal region of the protein, the IRs are buried within the protein and have little exposure to the surface. The IRs might be further hidden from surface exposure due to the possible dimerization of VlsE, forming a shield at the monomer-monomer interface.[5]These six IRs do not undergo changes during antigenic variation and are present in many strains and genospecies of B. burgdorferi[7].
, the second least exposed region composed of 26 amino acids (aa 274-298), has been found to be the most conserved IR and the most immunogenic[4] as observed in studies involving mice, monkeys and humans. In a study conducted by Liang et al., serum samples from monkeys, patients and mice all infected with Lyme disease were collected and the immunogenicity of each invariable region was tested. In monkeys, the results showed a robust response to IR6 and little to no response to the remaining IRs. Similarly, only IR6 elicited a strong immune response in infected humans with the exception of one patient also having a response to IR4. The immunodomminance of IR6 is depicted in Figure 3. In mice, however, a strong response was detected across IRs 6, 2, and 4, but not in IRs 1, 3, and 5[4], thus demonstrating the immunodominance of IR6 across species and indicating that further research into the immunogenicity of IRs 2 and 4 is needed. Due to its highly conserved structure and immunodominance across species, IR6 can clearly be seen as an important factor to the functionality of B. burgdorferi and therefore benefits from its strategic placement indicated in the crystallized structure.[5]
The high immunogenicity effect of IR6 is thought to only occur in dead bacteria due to its low maximal theoretical surface exposure in living B. borgderfuri (13.7%). Interaction with anti-IR6 antibodies would be limited to the exposed , Lys-274, Gln-279, Lys-291, and Lys-294.[5] These amino acids would be the likely targets of the host immune response. The immunodominant nature of VlsE, especially in IR6, makes it a viable diagnostic tool.[8]
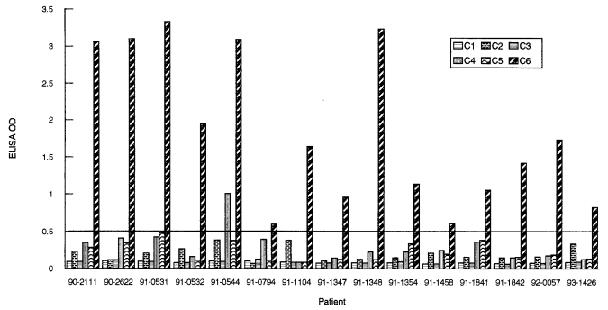
Figure 3 indicates that high immunogenicity of IR6. The Y-axis indicates the level of immunogenicity, the X-axis indicates each infected patient tested and each column within the graph indicates an invariable region, IR6 being the last column.
VlsE as a Diagnostic Tool
IR 6
IR6 is instrumental in the serodiagnosis of Lyme disease in its early stages. The sensitivity and precision of ELISA was measured based on a 26-mer synthetic peptide (C6) whose sequence matched that of IR6.[8] Serum samples in nonhuman primates that were introduced to different strains of B. burgdorferi were assessed for antibody responses. Antibody IgGwas present in high levels for all animals over a 160-week period following infection. Human patients assessed with the C6 ELISA yielded 74% detection sensitivity in the acute disease type, 90% in the convalescent (recovery) phase, 95% in the early disseminated phase, and 100% in late Lyme disease. No antibody responses to peptides resembling the sequences of the remaining IRs were detected in humans or monkeys.[9]
N- and C- Terminal Regions
In addition to the IR6 region, specific sequences of the N- and C- terminal invariable domains are known to be major B cell epitopes in Lyme disease patients. Although the N and C termini appeared to be flexible when crystallized[5], the crystal structure of VlsE indicates that the two sequences lie adjacent to each other, making them a single target covered by peptides VlsE21 through VlsE31 and VlsE336 through VlsE343.[10] It has been found that antibodies that interact with this membrane-proximal part become more potent in later stages of infection. According to ELISA results, post Lyme disease syndrome patients exhibited much higher antibody activity at the epitopes than fully recovered patients. Therefore, detection of these antibodies are useful in patient follow-ups, especially with those experiencing the later stages of Lyme disease.[10]
Conclusion
VlsE is thus an important virulence factor of B. burgdorferi. Through the gene conversion mechanism of the variable regions and the simultaneous protecting of the highly conserved invariable regions, the lipoprotein is able to effectively avoid the antibodies of the host immune system. These roles of VlsE are critical to the survival of the spirochete, as demonstrated by the decreased infectivity of B. burgdorferi upon the removal of the locus for VlsE.[6]
Though VlsE has been the object of intense scrutiny by researchers, there is still much to be understood. Future experiments may include further studies of the invariable regions, with a specific focus on IR6. IR6 is known to be highly conserved and thus, elicit immunogenic responses in the host once it is exposed. However, the function of IR6 for the survival of the spirochete is unknown. Focusing on how the invariable regions contribute to the virulence of B. borgdorferi may continue to increase our understanding of the function of VlsE.
Designing the Proteopedia page has greatly increased our understanding of VlsE. Through the process of creating green links to highlight the important functional aspects of VlsE’s structure, the process of relating the structural components of VlsE to its function became more coherent. Researching other articles that studied different aspects of VlsE also allowed us to better comprehend the function of this lipoprotein. In addition, working as a team helped to solidify certain concepts, as members of the group were able to help each other through the process of learning. Creating the Proteopedia webpage project has thus been a useful supplement in enhancing our understanding of VlsE.