SB2013 L04gr5
From Proteopedia
(→References) |
|||
| Line 21: | Line 21: | ||
Figure 2 shows the possible dimerization of VlsE. | Figure 2 shows the possible dimerization of VlsE. | ||
=Antigenic Variation= | =Antigenic Variation= | ||
| - | Antigenic variation is the process by which an organism is able to evade its host’s immune system. The antigenic variation that occurs in VlsE uses a complex genetic conversion mechanism that is seen to be concentrated in the VRs. The complex genetic system for VlsE is located near the right telomere on a 28-kb linear plasmid (lp28-1) in strains of ''B. burgdorferi''. This [http://en.wikipedia.org/wiki/Locus_%28genetics%29 locus] has been shown to be a crucial element for the persistence and virulence of Lyme disease (Bankhead and Chaconas 2007). The vls antigenic variation locus consists of a vls expression site (vlsE) and 15 silent vls cassettes that reside just upstream of the site. The vlsE cassette region, which is the variable domain and does not include the invariable amino or carboxyl termini, has approximately 92% DNA sequence identity with the silent vls cassettes. However, the silent vls cassettes lack promoter sequences and are therefore not expressed. Throughout the course of infection, the sequences for the flanking termini and the silent vls cassettes are conserved while the vlsE sequence is recombined. This suggests that the genetic variation mechanism occurs by copying segments of the 15 silent vls cassettes and completely replacing corresponding segments of vlsE sequences. The resulting differences are centralized in the highly variable regions of the vls cassettes. This allows for the constant evolution of the VR structures and evades the antibodies of the host immune system | + | Antigenic variation is the process by which an organism is able to evade its host’s immune system. The antigenic variation that occurs in VlsE uses a complex genetic conversion mechanism that is seen to be concentrated in the VRs. The complex genetic system for VlsE is located near the right telomere on a 28-kb linear plasmid (lp28-1) in strains of ''B. burgdorferi''. This [http://en.wikipedia.org/wiki/Locus_%28genetics%29 locus] has been shown to be a crucial element for the persistence and virulence of Lyme disease (Bankhead and Chaconas 2007). The ''vls'' antigenic variation locus consists of a ''vls'' expression site (''vlsE'') and 15 silent vls cassettes that reside just upstream of the site. The ''vlsE'' cassette region, which is the variable domain and does not include the invariable amino or carboxyl termini, has approximately 92% DNA sequence identity with the silent vls cassettes. However, the silent ''vls'' cassettes lack promoter sequences and are therefore not expressed. Throughout the course of infection, the sequences for the flanking termini and the silent ''vls'' cassettes are conserved while the vlsE sequence is recombined. This suggests that the genetic variation mechanism occurs by copying segments of the 15 silent vls cassettes and completely replacing corresponding segments of ''vlsE'' sequences. The resulting differences are centralized in the highly variable regions of the ''vls'' cassettes. This allows for the constant evolution of the VR structures and evades the antibodies of the host immune system <ref name="Zhang and Norris">. |
==''Variable Regions''== | ==''Variable Regions''== | ||
| Line 31: | Line 31: | ||
Interspersed in the variable regions of the variable domain are six invariable regions (IR1-IR6). Although located on the membrane distal region of the protein, the IRs are buried within the protein and have little exposure to the surface. The IRs might be further hidden from surface exposure due to the possible dimerization of VlsE, forming a shield at the monomer-monomer interface (Eicken et al 2002). These six IRs do not undergo changes during antigenic variation and are present in many strains and genospecies of ''B. burgdorferi''. | Interspersed in the variable regions of the variable domain are six invariable regions (IR1-IR6). Although located on the membrane distal region of the protein, the IRs are buried within the protein and have little exposure to the surface. The IRs might be further hidden from surface exposure due to the possible dimerization of VlsE, forming a shield at the monomer-monomer interface (Eicken et al 2002). These six IRs do not undergo changes during antigenic variation and are present in many strains and genospecies of ''B. burgdorferi''. | ||
| - | <scene name='SB2013_L04gr5/Ir6/1'>IR6</scene>, the second least exposed region composed of 26 amino acids, has been found to be the most conserved IR and the most immunogenic as observed in studies involving monkeys and humans. In a study conducted by Liang et al., serum samples from monkeys were infected by the bite of ''Ixodes scapularis'' nymphal ticks. The results showed a robust response to IR6 and little to no response to the remaining IRs. Similarly, only IR6 elicited a strong immune response in infected humans. Clearly, due to its highly conserved structure and immunodominance, IR6 is important to the functionality of ''B. | + | <scene name='SB2013_L04gr5/Ir6/1'>IR6</scene>, the second least exposed region composed of 26 amino acids, has been found to be the most conserved IR and the most immunogenic as observed in studies involving monkeys and humans. In a study conducted by Liang et al., serum samples from monkeys were infected by the bite of ''Ixodes scapularis'' nymphal ticks. The results showed a robust response to IR6 and little to no response to the remaining IRs. Similarly, only IR6 elicited a strong immune response in infected humans. Clearly, due to its highly conserved structure and immunodominance, IR6 is important to the functionality of ''B. burgdorferi'' and therefore requires its strategic placement indicated in the crystallized structure (Eicken et al 2002). In mice, however, a strong response was detected across IRs 6, 2, and 4, but not in IRs 1, 3, and 5. (Liang et al 1999 A), thus demonstrating the importance of IR6 across many species and indicating that further research into the fuctions of IRs 2 and 4 is needed. |
The high immunogenicity effect of IR6 is thought to only occur in dead bacteria due to its low maximal theoretical surface exposure in living ''B. borgderfuri'' (13.7%). Interaction with anti-IR6 antibodies would be limited to the exposed <scene name='SB2013_L04gr5/Ir6_residues/1'>amino acid residues</scene>, Lys-274, Gln-279, Lys-291,Lys-294 (Eicken). These amino acids would be the likely targets of the host immune response. The immunodominant nature of VlsE, especially in IR6, makes it a viable diagnostic tool.<ref name="Marangoni" /> | The high immunogenicity effect of IR6 is thought to only occur in dead bacteria due to its low maximal theoretical surface exposure in living ''B. borgderfuri'' (13.7%). Interaction with anti-IR6 antibodies would be limited to the exposed <scene name='SB2013_L04gr5/Ir6_residues/1'>amino acid residues</scene>, Lys-274, Gln-279, Lys-291,Lys-294 (Eicken). These amino acids would be the likely targets of the host immune response. The immunodominant nature of VlsE, especially in IR6, makes it a viable diagnostic tool.<ref name="Marangoni" /> | ||
| Line 65: | Line 65: | ||
9.<ref name="Liang B">Liang F, Steere A, Marques A, Johnson B, Miller J, Philipp M. 1999. Sensitive and Specific Serodiagnosis of Lyme Disease by Enzyme-Linked Immunosorbent Assay with a Peptide Based on an Immunodominant Conserved Region of ''Borrelia burgdorferi'' VlsE. Journal of Clinical Microbiology.37(12): 3990-3996. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/10565920 10565920]</ref> | 9.<ref name="Liang B">Liang F, Steere A, Marques A, Johnson B, Miller J, Philipp M. 1999. Sensitive and Specific Serodiagnosis of Lyme Disease by Enzyme-Linked Immunosorbent Assay with a Peptide Based on an Immunodominant Conserved Region of ''Borrelia burgdorferi'' VlsE. Journal of Clinical Microbiology.37(12): 3990-3996. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/10565920 10565920]</ref> | ||
10.<ref name="Chandra">Chandra A, Latov N, Wormser G, Marques A, Alaedini A. 2011. Epitope mapping of antibodies to VlsE protein of ''Borrelia burgdorferi'' in post-Lyme disease syndrome. Clinical Immunology. 141(1): 103-110. PMID[http://www.ncbi.nlm.nih.gov/pubmed/21778118 21778118]</ref> | 10.<ref name="Chandra">Chandra A, Latov N, Wormser G, Marques A, Alaedini A. 2011. Epitope mapping of antibodies to VlsE protein of ''Borrelia burgdorferi'' in post-Lyme disease syndrome. Clinical Immunology. 141(1): 103-110. PMID[http://www.ncbi.nlm.nih.gov/pubmed/21778118 21778118]</ref> | ||
| - | 11.<ref name="Marangoni">Marangoni A, Sambri V, Accardo S, Cavrini F, Mondardini V, Moroni A, Storni E, Cevenini R. 2006. A Decrease in the Immunoglobulin G Antibody Response Against against the VlsE Protein of Borrelia burgdorferi Sensu Lato Correlates with the Resolution of Clinical Signs in Antibiotic-Treated Patients with Early Lyme Disease. Clinical and Vaccine Immunology. 13(4): 525-529. PMID[http://www.ncbi.nlm.nih.gov/pubmed/16603623 16603623]</ref> | + | 11.<ref name="Marangoni">Marangoni A, Sambri V, Accardo S, Cavrini F, Mondardini V, Moroni A, Storni E, Cevenini R. 2006. A Decrease in the Immunoglobulin G Antibody Response Against against the VlsE Protein of ''Borrelia burgdorferi'' Sensu Lato Correlates with the Resolution of Clinical Signs in Antibiotic-Treated Patients with Early Lyme Disease. Clinical and Vaccine Immunology. 13(4): 525-529. PMID[http://www.ncbi.nlm.nih.gov/pubmed/16603623 16603623]</ref> |
</ref> | </ref> | ||
}} | }} | ||
Revision as of 15:34, 9 May 2013
VlsE
Lyme disease is the most pervasive tick-borne disease in Europe, the United States,and parts of Asia.[1] It is a multistage infection caused by the spirochete Borrelia burgdorferi. Early symptoms include headaches, depression, rash, and fever. If Lyme disease is left untreated, serious complications of the joints, heart and central nervous system can occur. Infected Ixodes ticks in their nymph stage transmit Lyme disease by attaching to humans and other mammals[2]. In most cases, the tick must be attached for 36-48 hours before the bacteria can be transmitted.[3]
Vmp-like sequence Expressed protein (VlsE), is a lipoprotein on the surface of Borrelia burgdorferi. VlsE contributes to the immune evasion and persistence of Lyme disease.[1] VlsE contains invariable and variable domains. It was found that the varaible domain is highly immunogenic and the target of the immune response; however, through antigenic variation the lipoprotein is able to evade the host immune system.[4]
Within the variable domain, there are invariable regions that remain unchanged during antigenic variation, and therefore may be targets of an immune response. IR6, the most conserved IR, has been found to be immunodominant. The antigencity of each of the 6 IRs has been studied using peptide-based enzyme linked immunosorbent essays, ELISA.[4]
Contents |
|
Structure
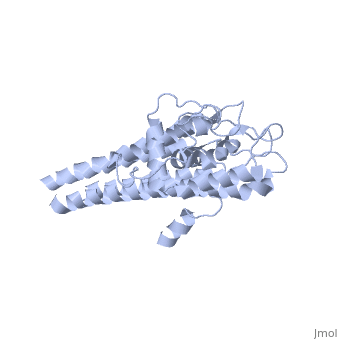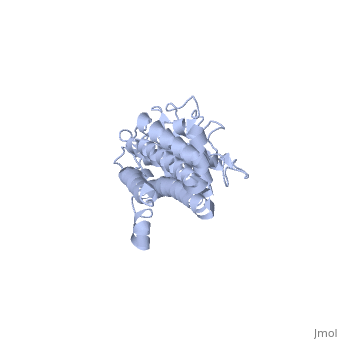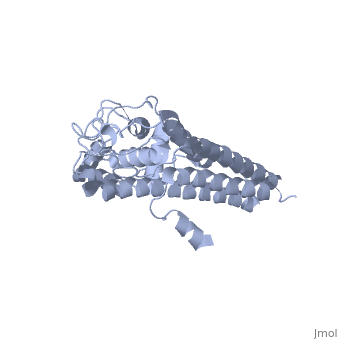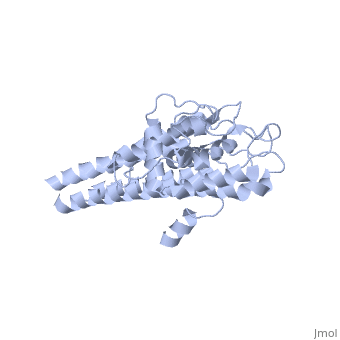
The lipoprotein, VlsE, consists of two invariable domains at the amino and carboxyl termini and a variable domain (Figure 1). When referring to the primary structure of the protein, the variable domain is a cassette region located between the two termini. The variable domain can be further broken down into variable regions () and invariable regions () (Liang et al. 1999).
When crystallized, VlsE forms a four molecule asymmetric unit with each molecule having slight differences in their conformation. Although each molecule in the unit is slightly different, a single molecule of the protein consists of eleven α-helices and four short β-strands. Helices α1 (aa 306-341), α2 (aa 68-87), α3 (aa 114-139), and α11 (aa 306-341) all form the of VlsE, while helices α4 through α10 form the primary region of the of the protein. The four short β-strands each consist of 3 amino acids and can also be located in the membrane distal region.
Covering the membrane distal part of VlsE are connecting loop regions, which lack secondary structure and have different conformations in each of the molecules. (Eicken et al. 2002). Helices α3 through α10 form the invariable regions and are attached by the connecting loops that are classified as the variable regions. Although VlsE crystallizes into an asymmetrical unit, it appears primarily as monomeric in solution. Because the interface between VlsE molecules in the crystal structure buries approximately 13% of the accessible surface area of the monomers, Eicken et al.suggest that there is a possibility of VlsE existing as a dimer when in its natural state (Figure 2).
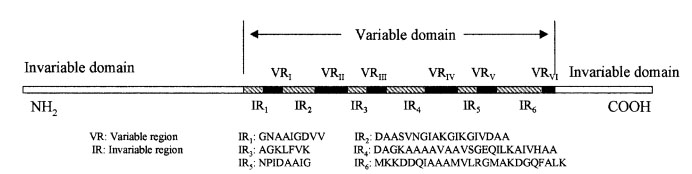 Figure 1 shows the different domains of VlsE.
Figure 1 shows the different domains of VlsE.
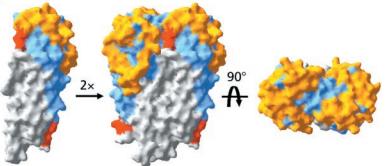 Figure 2 shows the possible dimerization of VlsE.
Figure 2 shows the possible dimerization of VlsE.
Antigenic Variation
Antigenic variation is the process by which an organism is able to evade its host’s immune system. The antigenic variation that occurs in VlsE uses a complex genetic conversion mechanism that is seen to be concentrated in the VRs. The complex genetic system for VlsE is located near the right telomere on a 28-kb linear plasmid (lp28-1) in strains of B. burgdorferi. This locus has been shown to be a crucial element for the persistence and virulence of Lyme disease (Bankhead and Chaconas 2007). The vls antigenic variation locus consists of a vls expression site (vlsE) and 15 silent vls cassettes that reside just upstream of the site. The vlsE cassette region, which is the variable domain and does not include the invariable amino or carboxyl termini, has approximately 92% DNA sequence identity with the silent vls cassettes. However, the silent vls cassettes lack promoter sequences and are therefore not expressed. Throughout the course of infection, the sequences for the flanking termini and the silent vls cassettes are conserved while the vlsE sequence is recombined. This suggests that the genetic variation mechanism occurs by copying segments of the 15 silent vls cassettes and completely replacing corresponding segments of vlsE sequences. The resulting differences are centralized in the highly variable regions of the vls cassettes. This allows for the constant evolution of the VR structures and evades the antibodies of the host immune system [5] 2.[2] 3.[3] 4.[4] 5.[6] 6.[5] 9.[7] 10.[8] 11.[9]
</ref>
}}
Proteopedia Page Contributors and Editors (what is this?)
Emma Brower, Julia Joseph, Alexandra DePastene, Olivia Rodrigues, Alexander Berchansky, Michal Harel