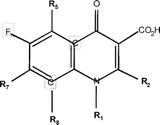
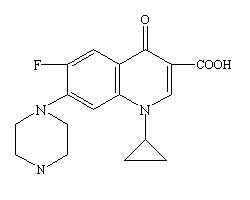
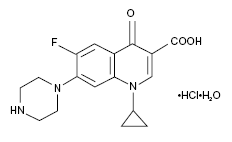
Ciprofloxacin
From Proteopedia
(Difference between revisions)
| Line 64: | Line 64: | ||
== Characteristic Protein Targets and Interactions == | == Characteristic Protein Targets and Interactions == | ||
| - | Ciprofloxacin is known for its efficient ability to hinder bacterial [[DNA]] synthesis via inhibition of bacterial [[DNA Gyrase]] and DNA [[Topoisomerase]] IV. <ref>Ciprofloxacin Oral - Monograph - Ciprofloxacin Hydrochloride. 2009. Medscape.com. http://www.medscape.com/druginfo/monograph cid=med&drugid=7748&drugname=Ciprofloxacin+Oral&monotype=monograph&secid=8.</ref>. DNA Gyrase, a type II DNA topoisomerase, is a tetramer composed of 2 GyrA and 2 GyrB subunits. DNA Gyrase is responsible for introducing negative superhelical twists | + | Ciprofloxacin is known for its efficient ability to hinder bacterial [[DNA]] synthesis via inhibition of bacterial [[DNA Gyrase]] and DNA [[Topoisomerase]] IV. <ref>Ciprofloxacin Oral - Monograph - Ciprofloxacin Hydrochloride. 2009. Medscape.com. http://www.medscape.com/druginfo/monograph cid=med&drugid=7748&drugname=Ciprofloxacin+Oral&monotype=monograph&secid=8.</ref>. DNA Gyrase, a type II DNA topoisomerase, is a tetramer composed of 2 GyrA subunits and 2 GyrB subunits. DNA Gyrase is responsible for introducing negative superhelical twists - as it removes positive superhelical twists - without which twists [[DNA Replication]] would not occur. Topoisomerase IV, also a type II DNA topoisomerase, is composed of 2 ParC subunits and 2 ParE subunits, and its overall structure is similar to that of DNA Gyrase. Specifically, ParC is homologous to GyrA, and ParE is homologous to GyrB. Topoisomerase IV is responsible for the separation of interlinked daughter chromosomes for the ultimate segregation of daughter cells. The action of Ciprofloxacin on DNA Gyrase and on Topoisomerase IV is characterized by the stabilization of DNA in complex with either of these two proteins. This stabilization prevents normal motility (and, thus, progression) of the DNA replication fork. This prevention leads to a full inhibition of DNA replication and, ultimately, to cell death. |
Revision as of 15:36, 26 June 2013
| |||||||||||
References
- ↑ CIPRO® (ciprofloxacin hydrochloride) TABLETS - CIPRO® (ciprofloxacin*) ORAL SUSPENSION - Drug Information Packet. Bayer HealthCare Pharmaceuticals. Schering Plough Corporation.
- ↑ CIPRO® (ciprofloxacin hydrochloride) TABLETS - CIPRO® (ciprofloxacin*) ORAL SUSPENSION - Drug Information Packet. Bayer HealthCare Pharmaceuticals. Schering Plough Corporation.
- ↑ 2011. Ciprofloxacin. Medicine Plus. American Society of Health-System Pharmacists Inc. 2011. http://www.nlm.nih.gov/medlineplus/druginfo/meds/a688016.html.
- ↑ 2001. Information on Cipro (Ciprofloxacin Hydrochloride) for Inhalation Anthrax for Consumers: Questions and Answers. Fda.gov. http://www.fda.gov/Drugs/EmergencyPreparedness/BioterrorismandDrugPreparedness/ucm130711.htm. Last updated, 2009.
- ↑ 2011. Ciprofloxacin. Medicine Plus. American Society of Health-System Pharmacists Inc. 2011. http://www.nlm.nih.gov/medlineplus/druginfo/meds/a688016.html.
- ↑ Ciprofloxacin. (2010). Pcm.me. http://pcm.me/ciprofloxacin/.
- ↑ Ciprofloxacin - Activity, Business Aspects/Bayer Pharmaceutical. Encyclopedia.jrank.org. http://encyclopedia.jrank.org/articles/pages/1398940/Ciprofloxacin.html
- ↑ Siegmund, K., et al. (2005). Molecular details of quinolone-DNA interactions: solution structure of an unusually stable DNA duplex with covalently linked nalidixic acid residues and non-covalent complexes derived from it. Nucleic Acids [Research], 33(15), 4838-4848.
- ↑ Peterson, L. (2001). Quinolone-Molecular Structure-Activity Relationships: What We Have Learned About Improving Antimicrobial Activity. Clinical Infectious Diseases, 33(3), S180-S186.
- ↑ Image from: http://cid.oxfordjournals.org/content/33/Supplement_3/S180.full.
- ↑ CIPRO® (ciprofloxacin hydrochloride) TABLETS - CIPRO® (ciprofloxacin*) ORAL SUSPENSION - Drug Information Packet. Bayer HealthCare Pharmaceuticals. Schering Plough Corporation.
- ↑ Molecular weight from Chemexper.com.
- ↑ CIPRO® (ciprofloxacin hydrochloride) TABLETS - CIPRO® (ciprofloxacin*) ORAL SUSPENSION - Drug Information Packet. Bayer HealthCare Pharmaceuticals. Schering Plough Corporation.
- ↑ Image from: http://www.google.com/imgres?imgurl=http://textbookofbacteriology.net/themicrobialworld/cipro.gif&imgrefurl=http://textbookofbacteriology.net/themicrobialworld/control.html&usg=__wtzKLHB3NssfnODEB224br5-Bcw=&h=200&w=250&sz=2&hl=en&start=0&zoom=1&tbnid=o7VT7s6FFIUrWM:&tbnh=160&tbnw=199&ei=Hk10TaypBcL58AbyvIjKDw&prev=/images%3Fq%3Dciprofloxacin%26um%3D1%26hl%3Den%26client%3Dfirefox-a%26sa%3DN%26rls%3Dorg.mozilla:en-US:official%26biw%3D1280%26bih%3D647%26tbs%3Disch:1&um=1&itbs=1&iact=hc&vpx=527&vpy=300&dur=1709&hovh=160&hovw=200&tx=155&ty=82&oei=EU10TcvOCMbdtge5msiLDw&page=1&ndsp=16&ved=1t:429,r:7,s:0.
- ↑ Molecular weight from: CIPRO® (ciprofloxacin hydrochloride) TABLETS - CIPRO® (ciprofloxacin*) ORAL SUSPENSION - Drug Information Packet. Bayer HealthCare Pharmaceuticals. Schering Plough Corporation.
- ↑ Image from: http://www.google.com/imgres?imgurl=http://images.rxlist.com/images/rxlist/ciloxan_s.gif&imgrefurl=http://www.rxlist.com/ciloxan_ophthalmic_ointment-drug.htm&usg=__UqTKseSe8hD85c5RLGIz2_dbAg0=&h=142&w=232&sz=2&hl=en&start=16&zoom=1&tbnid=70Q2WG5hppsQ5M:&tbnh=100&tbnw=164&ei=T010TenMFYH_8Aa6gvDKDw&prev=/images%3Fq%3Dciprofloxacin%26um%3D1%26hl%3Den%26client%3Dfirefox-a%26sa%3DN%26rls%3Dorg.mozilla:en-US:official%26biw%3D1280%26bih%3D647%26tbs%3Disch:10%2C624&um=1&itbs=1&iact=hc&vpx=1064&vpy=399&dur=309&hovh=106&hovw=174&tx=98&ty=76&oei=EU10TcvOCMbdtge5msiLDw&page=2&ndsp=18&ved=1t:429,r:17,s:16&biw=1280&bih=647.
- ↑ Ciprofloxacin. (2010). Pcm.me. http://pcm.me/ciprofloxacin/.
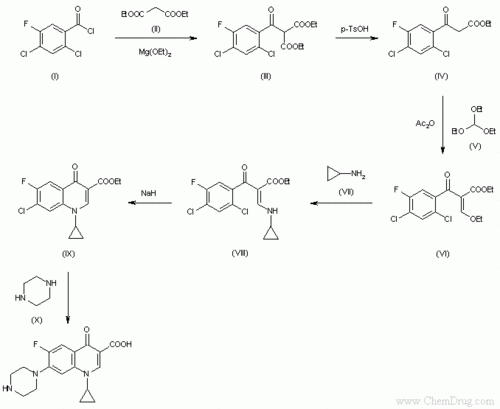
- ↑ Image from: http://www.google.com/imgres?imgurl=http://www.chemdrug.com/databases/SYNTHESIS/SYN/09/09000601a.gif&imgrefurl=http://www.chemdrug.com/databases/8_0_dvpytumicutbciwa.html&usg=__TxiDuzCve6C_crxmcPYTpfW5d4s=&h=555&w=678&sz=6&hl=en&start=0&zoom=1&tbnid=xhquLksJBbMnjM:&tbnh=165&tbnw=201&ei=0Y93TdbGI-yI0QGspa25Bw&prev=/images%3Fq%3Dsynthesis%2Bof%2Bciprofloxacin%26um%3D1%26hl%3Den%26client%3Dfirefox-a%26sa%3DN%26rls%3Dorg.mozilla:en-US:official%26biw%3D1280%26bih%3D647%26tbs%3Disch:1&um=1&itbs=1&iact=hc&vpx=346&vpy=105&dur=63&hovh=203&hovw=248&tx=170&ty=128&oei=0Y93TdbGI-yI0QGspa25Bw&page=1&ndsp=16&ved=1t:429,r:1,s:0
- ↑ Ciprofloxacin Oral - Monograph - Ciprofloxacin Hydrochloride. 2009. Medscape.com. http://www.medscape.com/druginfo/monograph cid=med&drugid=7748&drugname=Ciprofloxacin+Oral&monotype=monograph&secid=8.
- ↑ Su, Chih-Chia, et al. (2006). Conformation of the AcrB Multidrug Efflux Pump in Mutants of the Putative Proton Relay Pathway. Journal of Bacteriology, 188(20), 7290-7296.
- ↑ Husain, F., Nikaido, H. (2010). Substrate path in the AcrB multidrug efflux pump of Escherichia coli. Molecular Microbiology, 78(2), 320-330.
- ↑ Su, Chih-Chia, et al. (2006). Conformation of the AcrB Multidrug Efflux Pump in Mutants of the Putative Proton Relay Pathway. Journal of Bacteriology, 188(20), 7290-7296.
Proteopedia Page Contributors and Editors (what is this?)
John Ripollone, John E. Ripollone, Alexander Berchansky, David Canner