Ciprofloxacin
From Proteopedia
(Difference between revisions)
| Line 24: | Line 24: | ||
Most anaerobic bacteria exhibit Ciprofloxacin resistance. | Most anaerobic bacteria exhibit Ciprofloxacin resistance. | ||
| - | The effectiveness of Ciprofloxacin against the anthrax-causing bacteria, ''Bacillus anthracis'' has been demonstrated ''in vitro'' and by use of surrogate marker serum levels <ref>CIPRO® (ciprofloxacin hydrochloride) TABLETS - CIPRO® (ciprofloxacin*) ORAL SUSPENSION - Drug Information Packet. Bayer HealthCare Pharmaceuticals. Schering Plough Corporation.</ref><ref>2011. Ciprofloxacin. Medicine Plus. American Society of Health-System Pharmacists Inc. 2011. http://www.nlm.nih.gov/medlineplus/druginfo/meds/a688016.html.</ref>. Thus, Ciprofloxacin | + | The effectiveness of Ciprofloxacin against the anthrax-causing bacteria, ''Bacillus anthracis'' has been demonstrated ''in vitro'' and by use of surrogate marker serum levels <ref>CIPRO® (ciprofloxacin hydrochloride) TABLETS - CIPRO® (ciprofloxacin*) ORAL SUSPENSION - Drug Information Packet. Bayer HealthCare Pharmaceuticals. Schering Plough Corporation.</ref><ref>2011. Ciprofloxacin. Medicine Plus. American Society of Health-System Pharmacists Inc. 2011. http://www.nlm.nih.gov/medlineplus/druginfo/meds/a688016.html.</ref>. Thus, Ciprofloxacin, currently, is a Food and Drug Administration (FDA)-approved treatment for patients who have been exposed to anthrax via inhalation<ref>2001. Information on Cipro (Ciprofloxacin Hydrochloride) for Inhalation Anthrax for Consumers: Questions and Answers. Fda.gov. http://www.fda.gov/Drugs/EmergencyPreparedness/BioterrorismandDrugPreparedness/ucm130711.htm. Last updated, 2009.</ref>. Likewise, Ciprofloxacin may be used to treat plague (from the bacterium, ''Yersinia pestis'') and tularemia (from the bacterium, ''Francisella tularensis'')<ref>2011. Ciprofloxacin. Medicine Plus. American Society of Health-System Pharmacists Inc. 2011. http://www.nlm.nih.gov/medlineplus/druginfo/meds/a688016.html.</ref>. Thus, Ciprofloxacin demonstrates usefulness in the field of counter-bioterrorism given its action against bacteria that, potentially, could be implemented in biological warfare. Furthermore, in its extended-release tablet form, Ciprofloxacin targets certain types of urological infectious agents (e.g. those causing epididymitis). The nature of Ciprofloxacin, then, as a powerful, broad-range antibiotic is crucial for broad-range bacterial infection treatment. An understanding of the action of Ciprofloxacin at the molecular level is necessary for an appreciation of the potency of Ciprofloxacin witnessed at the macro level. |
== Historical Information == | == Historical Information == | ||
| - | + | Although the official patented introduction of Ciprofloxacin by Bayer Pharmaceuticals occurred in 1987, some reports indicated that at least two European patents for similar drugs pre-dated the Bayer patent by five years<ref>Ciprofloxacin. (2010). Pcm.me. http://pcm.me/ciprofloxacin/.</ref>. On October 27, 1987, the FDA approved the [[Pharmaceutical Drugs|drug]] for use in the United States for the treatment of certain bacterial infections. The effectiveness of Ciprofloxacin as an antibiotic went unchallenged by all alternative antibiotics<ref>Ciprofloxacin - Activity, Business Aspects/Bayer Pharmaceutical. Encyclopedia.jrank.org. http://encyclopedia.jrank.org/articles/pages/1398940/Ciprofloxacin.html</ref>. Consequently, other pharmaceutical companies were forced to offer their alternative antibiotics at lower costs (compared to the cost of Ciprofloxacin) so as to engage any sort of competition with Bayer Pharmaceuticals. Because of the increasingly common tendency of physicians to prescribe lower-cost medication, Bayer Pharmaceuticals could not expand into the international pharmaceutical market (which, as a whole, was declining) and, consequently, was forced to downsize at the turn of the century. Thus, the competitive effectiveness of Ciprofloxacin did not overcome the competitive pricing of drugs released by other pharmaceutical companies. Faced with the impending expiration of its patent for Ciprofloxacin in the early years of the millennium, Bayer Pharmaceuticals attempted to release variations of Ciprofloxacin. The release of Ciprofloxacin variations such as Pediatric Ciprofloxacin and Once-daily Ciprofloxacin allowed for the extension of the Ciprofloxacin patent. The popularity of Ciprofloxacin rose sharply after September 11, 2001 due to its characteristic targeting of ''Bacillus anthracis'', which was projected as a means of bioterrorism. The prescription of Ciprofloxacin for treatment of bacterial infections continues to this day. | |
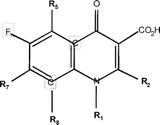
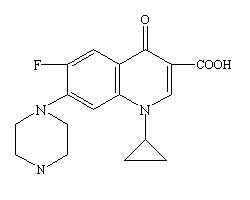
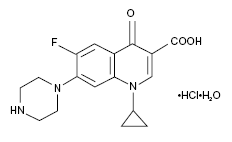
== Structure and Administration == | == Structure and Administration == | ||
Revision as of 15:06, 2 September 2013
| |||||||||||
References
- ↑ CIPRO® (ciprofloxacin hydrochloride) TABLETS - CIPRO® (ciprofloxacin*) ORAL SUSPENSION - Drug Information Packet. Bayer HealthCare Pharmaceuticals. Schering Plough Corporation.
- ↑ CIPRO® (ciprofloxacin hydrochloride) TABLETS - CIPRO® (ciprofloxacin*) ORAL SUSPENSION - Drug Information Packet. Bayer HealthCare Pharmaceuticals. Schering Plough Corporation.
- ↑ 2011. Ciprofloxacin. Medicine Plus. American Society of Health-System Pharmacists Inc. 2011. http://www.nlm.nih.gov/medlineplus/druginfo/meds/a688016.html.
- ↑ 2001. Information on Cipro (Ciprofloxacin Hydrochloride) for Inhalation Anthrax for Consumers: Questions and Answers. Fda.gov. http://www.fda.gov/Drugs/EmergencyPreparedness/BioterrorismandDrugPreparedness/ucm130711.htm. Last updated, 2009.
- ↑ 2011. Ciprofloxacin. Medicine Plus. American Society of Health-System Pharmacists Inc. 2011. http://www.nlm.nih.gov/medlineplus/druginfo/meds/a688016.html.
- ↑ Ciprofloxacin. (2010). Pcm.me. http://pcm.me/ciprofloxacin/.
- ↑ Ciprofloxacin - Activity, Business Aspects/Bayer Pharmaceutical. Encyclopedia.jrank.org. http://encyclopedia.jrank.org/articles/pages/1398940/Ciprofloxacin.html
- ↑ Siegmund, K., et al. (2005). Molecular details of quinolone-DNA interactions: solution structure of an unusually stable DNA duplex with covalently linked nalidixic acid residues and non-covalent complexes derived from it. Nucleic Acids [Research], 33(15), 4838-4848.
- ↑ Peterson, L. (2001). Quinolone-Molecular Structure-Activity Relationships: What We Have Learned About Improving Antimicrobial Activity. Clinical Infectious Diseases, 33(3), S180-S186.
- ↑ Image from: http://cid.oxfordjournals.org/content/33/Supplement_3/S180.full.
- ↑ CIPRO® (ciprofloxacin hydrochloride) TABLETS - CIPRO® (ciprofloxacin*) ORAL SUSPENSION - Drug Information Packet. Bayer HealthCare Pharmaceuticals. Schering Plough Corporation.
- ↑ Molecular weight from Chemexper.com.
- ↑ CIPRO® (ciprofloxacin hydrochloride) TABLETS - CIPRO® (ciprofloxacin*) ORAL SUSPENSION - Drug Information Packet. Bayer HealthCare Pharmaceuticals. Schering Plough Corporation.
- ↑ Image from: http://www.google.com/imgres?imgurl=http://textbookofbacteriology.net/themicrobialworld/cipro.gif&imgrefurl=http://textbookofbacteriology.net/themicrobialworld/control.html&usg=__wtzKLHB3NssfnODEB224br5-Bcw=&h=200&w=250&sz=2&hl=en&start=0&zoom=1&tbnid=o7VT7s6FFIUrWM:&tbnh=160&tbnw=199&ei=Hk10TaypBcL58AbyvIjKDw&prev=/images%3Fq%3Dciprofloxacin%26um%3D1%26hl%3Den%26client%3Dfirefox-a%26sa%3DN%26rls%3Dorg.mozilla:en-US:official%26biw%3D1280%26bih%3D647%26tbs%3Disch:1&um=1&itbs=1&iact=hc&vpx=527&vpy=300&dur=1709&hovh=160&hovw=200&tx=155&ty=82&oei=EU10TcvOCMbdtge5msiLDw&page=1&ndsp=16&ved=1t:429,r:7,s:0.
- ↑ Molecular weight from: CIPRO® (ciprofloxacin hydrochloride) TABLETS - CIPRO® (ciprofloxacin*) ORAL SUSPENSION - Drug Information Packet. Bayer HealthCare Pharmaceuticals. Schering Plough Corporation.
- ↑ Image from: http://www.google.com/imgres?imgurl=http://images.rxlist.com/images/rxlist/ciloxan_s.gif&imgrefurl=http://www.rxlist.com/ciloxan_ophthalmic_ointment-drug.htm&usg=__UqTKseSe8hD85c5RLGIz2_dbAg0=&h=142&w=232&sz=2&hl=en&start=16&zoom=1&tbnid=70Q2WG5hppsQ5M:&tbnh=100&tbnw=164&ei=T010TenMFYH_8Aa6gvDKDw&prev=/images%3Fq%3Dciprofloxacin%26um%3D1%26hl%3Den%26client%3Dfirefox-a%26sa%3DN%26rls%3Dorg.mozilla:en-US:official%26biw%3D1280%26bih%3D647%26tbs%3Disch:10%2C624&um=1&itbs=1&iact=hc&vpx=1064&vpy=399&dur=309&hovh=106&hovw=174&tx=98&ty=76&oei=EU10TcvOCMbdtge5msiLDw&page=2&ndsp=18&ved=1t:429,r:17,s:16&biw=1280&bih=647.
- ↑ Ciprofloxacin. (2010). Pcm.me. http://pcm.me/ciprofloxacin/.
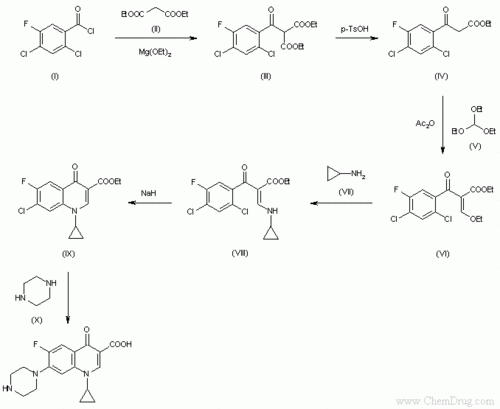
- ↑ Image from: http://www.google.com/imgres?imgurl=http://www.chemdrug.com/databases/SYNTHESIS/SYN/09/09000601a.gif&imgrefurl=http://www.chemdrug.com/databases/8_0_dvpytumicutbciwa.html&usg=__TxiDuzCve6C_crxmcPYTpfW5d4s=&h=555&w=678&sz=6&hl=en&start=0&zoom=1&tbnid=xhquLksJBbMnjM:&tbnh=165&tbnw=201&ei=0Y93TdbGI-yI0QGspa25Bw&prev=/images%3Fq%3Dsynthesis%2Bof%2Bciprofloxacin%26um%3D1%26hl%3Den%26client%3Dfirefox-a%26sa%3DN%26rls%3Dorg.mozilla:en-US:official%26biw%3D1280%26bih%3D647%26tbs%3Disch:1&um=1&itbs=1&iact=hc&vpx=346&vpy=105&dur=63&hovh=203&hovw=248&tx=170&ty=128&oei=0Y93TdbGI-yI0QGspa25Bw&page=1&ndsp=16&ved=1t:429,r:1,s:0
- ↑ Ciprofloxacin Oral - Monograph - Ciprofloxacin Hydrochloride. 2009. Medscape.com. http://www.medscape.com/druginfo/monograph cid=med&drugid=7748&drugname=Ciprofloxacin+Oral&monotype=monograph&secid=8.
- ↑ Su, Chih-Chia, et al. (2006). Conformation of the AcrB Multidrug Efflux Pump in Mutants of the Putative Proton Relay Pathway. Journal of Bacteriology, 188(20), 7290-7296.
- ↑ Husain, F., Nikaido, H. (2010). Substrate path in the AcrB multidrug efflux pump of Escherichia coli. Molecular Microbiology, 78(2), 320-330.
- ↑ Su, Chih-Chia, et al. (2006). Conformation of the AcrB Multidrug Efflux Pump in Mutants of the Putative Proton Relay Pathway. Journal of Bacteriology, 188(20), 7290-7296.
Proteopedia Page Contributors and Editors (what is this?)
John Ripollone, John E. Ripollone, Alexander Berchansky, David Canner