We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
NF-Y Transcription Factor Sandbox
From Proteopedia
(Difference between revisions)
| Line 9: | Line 9: | ||
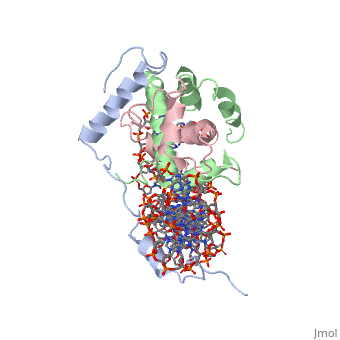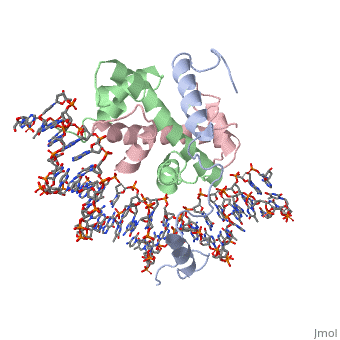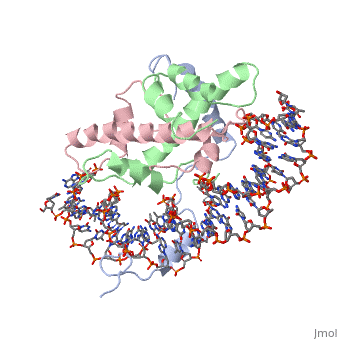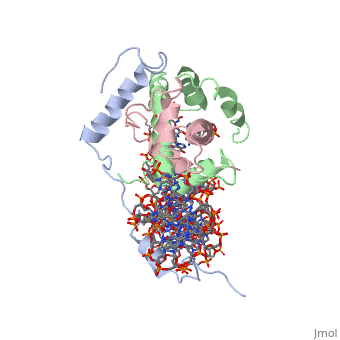
<scene name='56/566534/Nf-yb_real/1'>NF-YB</scene>, and <scene name='56/566534/Nf-yc_real/1'>NF-YC</scene> subunits. NF-YA subunit contains two α-helices, NF-YB subunit contains four α-helices and two β-sheets, and NF-YC subunit contains three α-helices and two β-sheets. The NF-YB and NF-YC subunits each contain a histone fold motif and form a NF-YB/NF-YC histone folding domain (HFD) dimer<ref>PMID: 24030830</ref>. The composition of mostly α-helices gives the protein flexibility. One of the two α helices of the NF-YA subunit, the N terminal <scene name='56/566534/Nf-ya_a1_helix/1'>A1 helix</scene>, interacts with NF-YB/NF-YC heterodimer resulting in a heterotrimer. | <scene name='56/566534/Nf-yb_real/1'>NF-YB</scene>, and <scene name='56/566534/Nf-yc_real/1'>NF-YC</scene> subunits. NF-YA subunit contains two α-helices, NF-YB subunit contains four α-helices and two β-sheets, and NF-YC subunit contains three α-helices and two β-sheets. The NF-YB and NF-YC subunits each contain a histone fold motif and form a NF-YB/NF-YC histone folding domain (HFD) dimer<ref>PMID: 24030830</ref>. The composition of mostly α-helices gives the protein flexibility. One of the two α helices of the NF-YA subunit, the N terminal <scene name='56/566534/Nf-ya_a1_helix/1'>A1 helix</scene>, interacts with NF-YB/NF-YC heterodimer resulting in a heterotrimer. | ||
| - | <br>The NF-Y heterotrimer is stabilized by ionic interactions, interactions between the backbone atoms of residues, and hydrophobic residues. Stabilizing ionic interactions occur between Asn239(NF-YA) with Asp109(NF-YC) and Asp112(NF-YC)<ref name="mainarticle" />. Residue backbone interactions occur between Leu123(NF-YB) with Phe113(NF-YC), Arg245(NF-YA) with Glu98(NF-YB) and Glu101(NF-YB), Arg249(NF-YA) with Glu90(NF-YB), and Arg250(NF-YA) with Asp116(NF-YC)<ref name="mainarticle" />. <scene name='56/566534/Hydrophobic_residues/1'>Hydrophobic residues</scene> that contribute to the stabilization of the NF-Y heterotrimer are only located at NF-YA and NF-YB subunits at residues Ile246(NF-YA), Phe94(NF-YB), and Ile115(NF-YB)<ref name="mainarticle" />({{Template:ColorKey_Hydrophobic}}{{Template:ColorKey_Polar}}). The NF-Y heterotrimer is also stabilized by the <scene name='56/566534/A1a2_linker/2'>A1A2 linker</scene> segment through intramolecular interactions of NF-YA residues on the main chain and side chain. Along with stabilization, the A1A2 linker provides the flexibility needed to direct the NF-YA chain toward DNA<ref name="mainarticle" />. | + | <br>The NF-Y heterotrimer is stabilized by ionic interactions, interactions between the backbone atoms of residues, and hydrophobic residues. Stabilizing ionic interactions occur between Asn239(NF-YA) with Asp109(NF-YC) and Asp112(NF-YC)<ref name="mainarticle" />. Residue backbone interactions occur between Leu123(NF-YB) with Phe113(NF-YC), Arg245(NF-YA) with Glu98(NF-YB) and Glu101(NF-YB), Arg249(NF-YA) with Glu90(NF-YB), and Arg250(NF-YA) with Asp116(NF-YC)<ref name="mainarticle" />. <scene name='56/566534/Hydrophobic_residues/1'>Hydrophobic residues</scene> that contribute to the stabilization of the NF-Y heterotrimer are only located at NF-YA and NF-YB subunits at residues Ile246(NF-YA), Phe94(NF-YB), and Ile115(NF-YB)<ref name="mainarticle" />({{Template:ColorKey_Hydrophobic}} {{Template:ColorKey_Polar}}). The NF-Y heterotrimer is also stabilized by the <scene name='56/566534/A1a2_linker/2'>A1A2 linker</scene> segment through intramolecular interactions of NF-YA residues on the main chain and side chain. Along with stabilization, the A1A2 linker provides the flexibility needed to direct the NF-YA chain toward DNA<ref name="mainarticle" />. |
<br>Furthermore, the NF-Y gene can be deferentially spliced to provide different isoforms of the protein. <ref name="activation" />. For example, NF-YA has two isoforms, which differ in the amount of amino acids in the glutamine (Q)-rich activation domain<ref name="activation">PMID: 22050321</ref>. The purpose of these isoforms has yet to be seen, however studies suggest that certain gene expression is dependent on which isoform is present at a time<ref name="activation" />. Another study showed that NF-YA and NF-YB is required for embryonic stem cell (ESC) viability<ref name="activation" />. | <br>Furthermore, the NF-Y gene can be deferentially spliced to provide different isoforms of the protein. <ref name="activation" />. For example, NF-YA has two isoforms, which differ in the amount of amino acids in the glutamine (Q)-rich activation domain<ref name="activation">PMID: 22050321</ref>. The purpose of these isoforms has yet to be seen, however studies suggest that certain gene expression is dependent on which isoform is present at a time<ref name="activation" />. Another study showed that NF-YA and NF-YB is required for embryonic stem cell (ESC) viability<ref name="activation" />. | ||
| Line 20: | Line 20: | ||
===DNA Interaction=== | ===DNA Interaction=== | ||
| - | NF-Y interacts with DNA in several ways; one particular way is by using the C terminal <scene name='56/566534/Nf-ya_a2_helix_in_minor_groo/1'>A2 helix</scene> of the NF-YA subunit inserts deep into the minor groove of DNA. NF-YA A2 helix binds to the <scene name='56/566534/Ccaat_box/4'>CCAAT</scene> box and causes the minor groove to widen at the CCAAT box<ref name="mainarticle" />. Van der Waals and <scene name='56/566534/Nf-y_dna_complex/1'>electrostatic interactions</scene> provide the stabilization of the NF-Y/DNA complex due to the highly basic surface of the NF-YB/NF-YC HFD dimer and negatively charged DNA<ref name="mainarticle" />({{Template:ColorKey_Hydrophobic}} | + | NF-Y interacts with DNA in several ways; one particular way is by using the C terminal <scene name='56/566534/Nf-ya_a2_helix_in_minor_groo/1'>A2 helix</scene> of the NF-YA subunit inserts deep into the minor groove of DNA. NF-YA A2 helix binds to the <scene name='56/566534/Ccaat_box/4'>CCAAT</scene> box and causes the minor groove to widen at the CCAAT box<ref name="mainarticle" />. Van der Waals and <scene name='56/566534/Nf-y_dna_complex/1'>electrostatic interactions</scene> provide the stabilization of the NF-Y/DNA complex due to the highly basic surface of the NF-YB/NF-YC HFD dimer and negatively charged DNA<ref name="mainarticle" />({{Template:ColorKey_Hydrophobic}} {{Template:ColorKey_Polar}}). |
| - | {{Template:ColorKey_Polar}}). | + | |
== References == | == References == | ||
<references /> | <references /> | ||
Revision as of 05:31, 7 November 2013
| |||||||||||