Sandbox Reserved 771
From Proteopedia
| Line 44: | Line 44: | ||
===Glycine Triad=== | ===Glycine Triad=== | ||
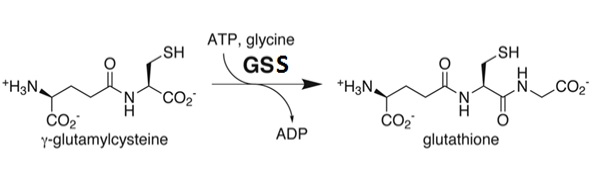
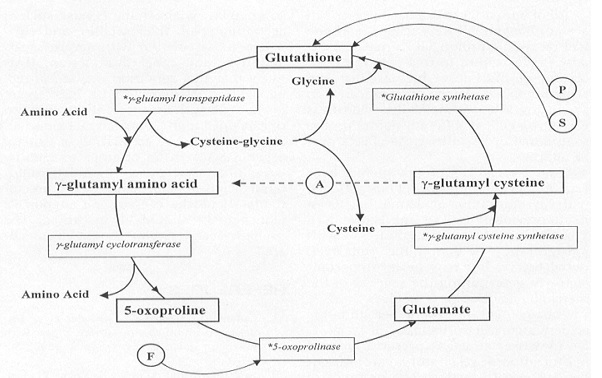
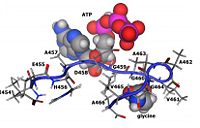
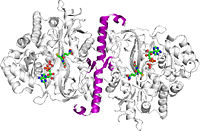
| - | As stated previously, the catalytic active site of GSS is composed of the G-loop, S-loop, and A-loop. The G-loop has been termed the “<scene name='56/564047/Glycine_triad/1 | + | As stated previously, the catalytic active site of GSS is composed of the G-loop, S-loop, and A-loop. The G-loop has been termed the “<scene name='56/564047/Glycine_triad/1'>Glycine Triad</scene>” due to the contribution of three glycine residues in this loop to the enzymatic activity of GSS – Gly369, Gly370, and Gly371. While all three residues are essential to the activity of the enzyme, kinetic experiments have shown Gly369 and Gly370 to have much more critical roles than Gly371. G369V and G370V variants were found to contain a mere 0.7% and 0.3% of the activity of the wild type GSS enzyme, respectively. G371V mutants still contained approximately 13% of the wild type activity, indicating a level of importance similar to the Asp458 residue of the A-loop. These experimental results suggest that the mechanism of activity interference lies in a decreased ligand binding and failure to close the active site once the ligand has bound. |
Revision as of 01:25, 5 December 2013
| This Sandbox is Reserved from Sep 25, 2013, through Mar 31, 2014 for use in the course "BCH455/555 Proteins and Molecular Mechanisms" taught by Michael B. Goshe at the North Carolina State University. This reservation includes Sandbox Reserved 299, Sandbox Reserved 300 and Sandbox Reserved 760 through Sandbox Reserved 779. |
To get started:
More help: Help:Editing |
| |||||||||||
References
1. http://www.ncbi.nlm.nih.gov/protein/NP_000169.1
2. Breton CV, Salam MT, Vora H, Gauderman WJ, Gilliland FD. 2011. Genetic variation in the glutathione synthesis pathway, air pollution, and children's lung function growth. Amer Jour Respir Crit Care Med, 183(2): 243-248. doi: 10.1164/rccm.201006-0849OC
3. Brown TR, Drummond ML, Barelier S, Crutchfield AS, Dinescu A, Slavens KD, Cundari TR, Anderson ME. 2011. Asparate 458 of human glutathione synthetase is importatnt for cooperativity and active site structure. Biochem & Biophys Resear Comm, 411(3): 536-542. doi: 10.1016/j.bbrc.2011.06.166
4. Dinescu A, Brown TR, Barelier S, Cundari TR, Anderson ME. 2010. The role of the glycine triad in human glutathione synthesis. Biochem Biophys Res Commun, 400(4):511-516. doi: 10.1016/j.bbrc.2010.08.081
5. Slavens KD, Brown TR, Barakat KA, Cundari TR, Anderson ME. 2011. Valine 44 and valine 45 of human glutathione synthetase are key for subunit stability and negative cooperativity. Biochem & Biophys Resear Comm, 410(3): 597-601. doi: 10.1016/j.bbrc.2011.06.034
6. Uchida M, Sugaya M, Janamary T, Hisatomi H. 2010. Alternative RNA splicing in expression of the glutathione synthetase gene in human cells. Mol Biol Rep, 37(4): 2105-2109. doi: 10.1007/s11033-009-9675-3