We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 765
From Proteopedia
(Difference between revisions)
| Line 46: | Line 46: | ||
==Mechanism== | ==Mechanism== | ||
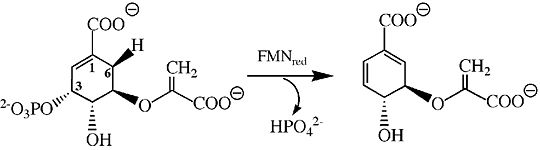
| - | Below is the proposed mechanism for the enzyme chorismate synthase. Since chorismate synthase is part of the lyase family, there will be an elimination reaction with a newly formed double bond. A very important cofactor is needed, Flavin Mononucleotide (FMN), when it is reduced the reaction is able to take place. The predicted model is that <scene name='56/564041/His_106/2'>HIS 106</scene> protonates the monoanionic reduced FMN and then <scene name='56/564041/His_17/2'>HIS 17</scene> protonates the leaving inorganic phosphate group of the substrate <ref>PMID:14668332</ref>. This is consider to be the <scene name='56/564041/Active_site/3'>active site</scene>, where all of the catalytic events partake. Based on current studies, FMN comes in and is protonated immediately by HIS 106 making FMNH2 and leaving. His 17 then protonates the leaving phosphate group to allow for the elimination to be completed <ref>PMID:14668332</ref>. To finish off the reaction, the electrons are shifted around to make a double bond that is necessary for the synthesis of aromatic amino acids. The aromatic amino acids that are form are tyrosine, tyrptophan, and phenylalanine; because of the double bond being formed within the six-membered ring. | + | Below is the proposed mechanism for the enzyme chorismate synthase. Since chorismate synthase is part of the lyase family, there will be an elimination reaction with a newly formed double bond. A very important cofactor is needed, Flavin Mononucleotide (FMN), when it is reduced the reaction is able to take place <ref>PMID:9951731</ref>. The predicted model is that <scene name='56/564041/His_106/2'>HIS 106</scene> protonates the monoanionic reduced FMN and then <scene name='56/564041/His_17/2'>HIS 17</scene> protonates the leaving inorganic phosphate group of the substrate <ref>PMID:14668332</ref>. This is consider to be the <scene name='56/564041/Active_site/3'>active site</scene>, where all of the catalytic events partake. Based on current studies, FMN comes in and is protonated immediately by HIS 106 making FMNH2 and leaving. His 17 then protonates the leaving phosphate group to allow for the elimination to be completed <ref>PMID:14668332</ref>. To finish off the reaction, the electrons are shifted around to make a double bond that is necessary for the synthesis of aromatic amino acids. The aromatic amino acids that are form are tyrosine, tyrptophan, and phenylalanine; because of the double bond being formed within the six-membered ring. |
[[Image:Chorismate Synthase Mechanism.jpg|thumb|540px|Figure 3. The main mechanism of chorismate synthase involving HIS 17.]] | [[Image:Chorismate Synthase Mechanism.jpg|thumb|540px|Figure 3. The main mechanism of chorismate synthase involving HIS 17.]] | ||
Revision as of 03:27, 7 December 2013
| This Sandbox is Reserved from Sep 25, 2013, through Mar 31, 2014 for use in the course "BCH455/555 Proteins and Molecular Mechanisms" taught by Michael B. Goshe at the North Carolina State University. This reservation includes Sandbox Reserved 299, Sandbox Reserved 300 and Sandbox Reserved 760 through Sandbox Reserved 779. |
To get started:
More help: Help:Editing |
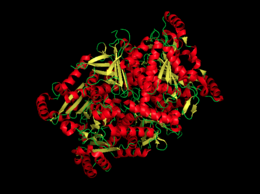
Chorismate Synthase
| |||||||||||