2vab
From Proteopedia
(New page: 200px<br /> <applet load="2vab" size="450" color="white" frame="true" align="right" spinBox="true" caption="2vab, resolution 2.5Å" /> '''MHC CLASS I H-2KB HE...) |
|||
| Line 1: | Line 1: | ||
| - | [[Image:2vab.gif|left|200px]]<br /> | + | [[Image:2vab.gif|left|200px]]<br /><applet load="2vab" size="350" color="white" frame="true" align="right" spinBox="true" |
| - | <applet load="2vab" size=" | + | |
caption="2vab, resolution 2.5Å" /> | caption="2vab, resolution 2.5Å" /> | ||
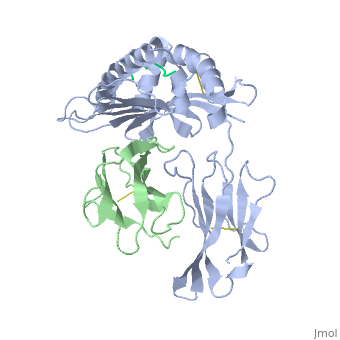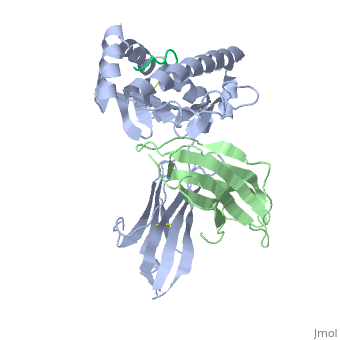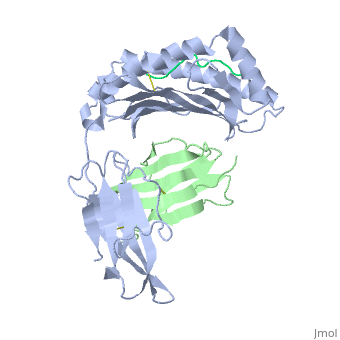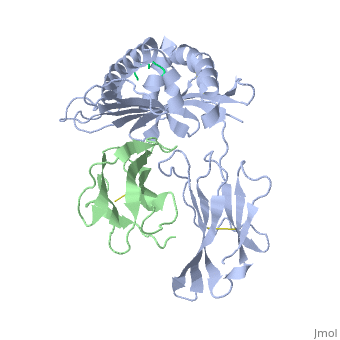
'''MHC CLASS I H-2KB HEAVY CHAIN COMPLEXED WITH BETA-2 MICROGLOBULIN AND SENDAI VIRUS NUCLEOPROTEIN'''<br /> | '''MHC CLASS I H-2KB HEAVY CHAIN COMPLEXED WITH BETA-2 MICROGLOBULIN AND SENDAI VIRUS NUCLEOPROTEIN'''<br /> | ||
==Overview== | ==Overview== | ||
| - | The x-ray structures of a murine MHC class I molecule (H-2Kb) were | + | The x-ray structures of a murine MHC class I molecule (H-2Kb) were determined in complex with two different viral peptides, derived from the vesicular stomatitis virus nucleoprotein (52-59), VSV-8, and the Sendai virus nucleoprotein (324-332), SEV-9. The H-2Kb complexes were refined at 2.3 A for VSV-8 and 2.5 A for SEV-9. The structure of H-2Kb exhibits a high degree of similarity with human HLA class I, although the individual domains can have slightly altered dispositions. Both peptides bind in extended conformations with most of their surfaces buried in the H-2Kb binding groove. The nonamer peptide maintains the same amino- and carboxyl-terminal interactions as the octamer primarily by the insertion of a bulge in the center of an otherwise beta conformation. Most of the specific interactions are between side-chain atoms of H-2Kb and main-chain atoms of peptide. This binding scheme accounts in large part for the enormous diversity of peptide sequences that bind with high affinity to class I molecules. Small but significant conformational changes in H-2Kb are associated with peptide binding, and these synergistic movements may be an integral part of the T cell receptor recognition process. |
==About this Structure== | ==About this Structure== | ||
| - | 2VAB is a [http://en.wikipedia.org/wiki/Protein_complex Protein complex] structure of sequences from [http://en.wikipedia.org/wiki/Mus_musculus Mus musculus] and [http://en.wikipedia.org/wiki/Sendai_virus Sendai virus]. This structure | + | 2VAB is a [http://en.wikipedia.org/wiki/Protein_complex Protein complex] structure of sequences from [http://en.wikipedia.org/wiki/Mus_musculus Mus musculus] and [http://en.wikipedia.org/wiki/Sendai_virus Sendai virus]. This structure supersedes the now removed PDB entry 1VAB. The following page contains interesting information on the relation of 2VAB with [[http://pdb.rcsb.org/pdb/static.do?p=education_discussion/molecule_of_the_month/pdb62_1.html Major Histocompatibility Complex]]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=2VAB OCA]. |
==Reference== | ==Reference== | ||
| Line 16: | Line 15: | ||
[[Category: Protein complex]] | [[Category: Protein complex]] | ||
[[Category: Sendai virus]] | [[Category: Sendai virus]] | ||
| - | [[Category: Fremont, D | + | [[Category: Fremont, D H.]] |
| - | [[Category: Wilson, I | + | [[Category: Wilson, I A.]] |
[[Category: class i major histocompatibility complex]] | [[Category: class i major histocompatibility complex]] | ||
[[Category: histocompatibility antigen]] | [[Category: histocompatibility antigen]] | ||
| Line 23: | Line 22: | ||
[[Category: mhc-peptide complex]] | [[Category: mhc-peptide complex]] | ||
| - | ''Page seeded by [http:// | + | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Thu Feb 21 18:54:32 2008'' |
Revision as of 16:54, 21 February 2008
|
MHC CLASS I H-2KB HEAVY CHAIN COMPLEXED WITH BETA-2 MICROGLOBULIN AND SENDAI VIRUS NUCLEOPROTEIN
Overview
The x-ray structures of a murine MHC class I molecule (H-2Kb) were determined in complex with two different viral peptides, derived from the vesicular stomatitis virus nucleoprotein (52-59), VSV-8, and the Sendai virus nucleoprotein (324-332), SEV-9. The H-2Kb complexes were refined at 2.3 A for VSV-8 and 2.5 A for SEV-9. The structure of H-2Kb exhibits a high degree of similarity with human HLA class I, although the individual domains can have slightly altered dispositions. Both peptides bind in extended conformations with most of their surfaces buried in the H-2Kb binding groove. The nonamer peptide maintains the same amino- and carboxyl-terminal interactions as the octamer primarily by the insertion of a bulge in the center of an otherwise beta conformation. Most of the specific interactions are between side-chain atoms of H-2Kb and main-chain atoms of peptide. This binding scheme accounts in large part for the enormous diversity of peptide sequences that bind with high affinity to class I molecules. Small but significant conformational changes in H-2Kb are associated with peptide binding, and these synergistic movements may be an integral part of the T cell receptor recognition process.
About this Structure
2VAB is a Protein complex structure of sequences from Mus musculus and Sendai virus. This structure supersedes the now removed PDB entry 1VAB. The following page contains interesting information on the relation of 2VAB with [Major Histocompatibility Complex]. Full crystallographic information is available from OCA.
Reference
Crystal structures of two viral peptides in complex with murine MHC class I H-2Kb., Fremont DH, Matsumura M, Stura EA, Peterson PA, Wilson IA, Science. 1992 Aug 14;257(5072):919-27. PMID:1323877
Page seeded by OCA on Thu Feb 21 18:54:32 2008