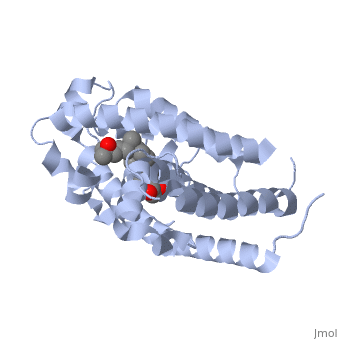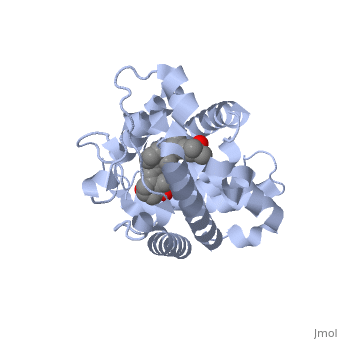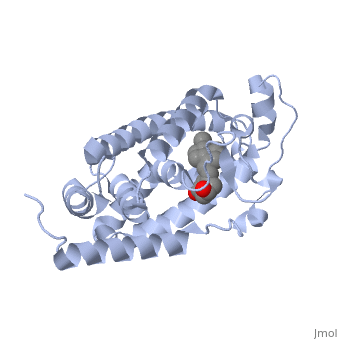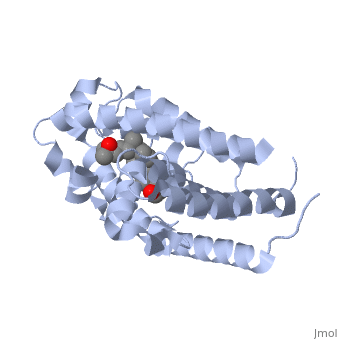
Vitamin D receptor
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | [[Image:1db1.png|left|200px|thumb|Crystal structure of human vitamin D receptor ligand-binding domain complex with vitamin D [[1db1]]]] | ||
<StructureSection load='1db1' size='350' side='right' caption='Structure of human vitamin D receptor ligand-binding domain complex with vitamin D (PDB entry [[1db1]])' scene=''> | <StructureSection load='1db1' size='350' side='right' caption='Structure of human vitamin D receptor ligand-binding domain complex with vitamin D (PDB entry [[1db1]])' scene=''> | ||
| Line 26: | Line 25: | ||
<scene name='56/562378/Serine_final/1'>Serine</scene> is replaced with <scene name='56/562378/Asparticacid_final/1'>aspartic acid</scene> when mutated creating a negative charge. The negative charge at the residue inhibits DNA binding which cause a down – regulation of VDR activity. VDR needs DNA binding in order for it to be activated which is only possible with a serine residue. Research is still continuing to find a therapeutic cause for this mutation. | <scene name='56/562378/Serine_final/1'>Serine</scene> is replaced with <scene name='56/562378/Asparticacid_final/1'>aspartic acid</scene> when mutated creating a negative charge. The negative charge at the residue inhibits DNA binding which cause a down – regulation of VDR activity. VDR needs DNA binding in order for it to be activated which is only possible with a serine residue. Research is still continuing to find a therapeutic cause for this mutation. | ||
| - | </StructureSection> | ||
| - | |||
| + | <!-- | ||
==Crystal structure of the human VDR ligand binding domain bound to the synthetic agonist compound 2alpha-methyl-AMCR277A(C23S)== | ==Crystal structure of the human VDR ligand binding domain bound to the synthetic agonist compound 2alpha-methyl-AMCR277A(C23S)== | ||
| - | <StructureSection load='3a3z' size='350' side='right' caption='Synthetic agonist (PDB entry [[3a3z]])' scene=''>The vitamin D nuclear receptor is a ligand-dependent transcription factor that controls multiple biological responses such as cell proliferation, immune responses, and bone mineralization. Numerous 1 alpha,25(OH)(2)D(3) analogues, which exhibit low calcemic side effects and/or antitumoral properties, have been synthesized. In the article, "Structure-function relationships and crystal structures of the vitamin D receptor bound 2 alpha-methyl-(20S,23S)- and 2 alpha-methyl-(20S,23R)-epoxymethano-1 alpha,25-dihydroxyvitamin D3" by Antony, P. et al, they showed that <scene name='56/562378/3a3z/1'>the synthetic analogue (20S,23S)-epoxymethano-1alpha,25-dihydroxyvitamin D(3) (2a)</scene> acts as a 1alpha,25(OH)(2)D(3) superagonist and exhibits both antiproliferative and prodifferentiating properties in vitro. Using this information and on the basis of the crystal structures of human VDR ligand binding domain (hVDR LBD) bound to 1alpha,25(OH)(2)D(3), 2alpha-methyl-1alpha,25(OH)(2)D(3), or 2a, we designed a novel analogue, 2alpha-methyl-(20S,23S)-epoxymethano-1alpha,25-dihydroxyvitamin D(3) (4a), in order to increase its transactivation potency. Here, we solved the crystal structures of the hVDR LBD in complex with the 4a (C23S) and its epimer 4b (C23R) and determined their correlation with specific biological outcomes. | + | <StructureSection load='3a3z' size='350' side='right' caption='Synthetic agonist (PDB entry [[3a3z]])' scene=''> |
| - | + | --> | |
| + | The vitamin D nuclear receptor is a ligand-dependent transcription factor that controls multiple biological responses such as cell proliferation, immune responses, and bone mineralization. Numerous 1 alpha,25(OH)(2)D(3) analogues, which exhibit low calcemic side effects and/or antitumoral properties, have been synthesized. In the article, "Structure-function relationships and crystal structures of the vitamin D receptor bound 2 alpha-methyl-(20S,23S)- and 2 alpha-methyl-(20S,23R)-epoxymethano-1 alpha,25-dihydroxyvitamin D3" by Antony, P. et al, they showed that <scene name='56/562378/3a3z/1'>the synthetic analogue (20S,23S)-epoxymethano-1alpha,25-dihydroxyvitamin D(3) (2a)</scene> acts as a 1alpha,25(OH)(2)D(3) superagonist and exhibits both antiproliferative and prodifferentiating properties in vitro. Using this information and on the basis of the crystal structures of human VDR ligand binding domain (hVDR LBD) bound to 1alpha,25(OH)(2)D(3), 2alpha-methyl-1alpha,25(OH)(2)D(3), or 2a, we designed a novel analogue, 2alpha-methyl-(20S,23S)-epoxymethano-1alpha,25-dihydroxyvitamin D(3) (4a), in order to increase its transactivation potency. Here, we solved the crystal structures of the hVDR LBD in complex with the 4a (C23S) and its epimer 4b (C23R) and determined their correlation with specific biological outcomes. | ||
Revision as of 15:35, 3 February 2014
| |||||||||||
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Jaime Prilusky, Isita Amin