Vitamin D receptor
From Proteopedia
(Difference between revisions)
| Line 3: | Line 3: | ||
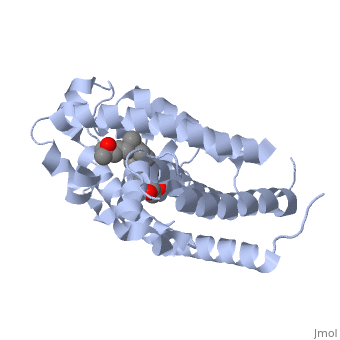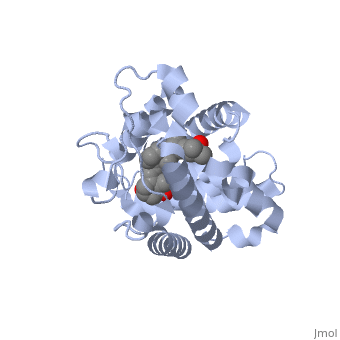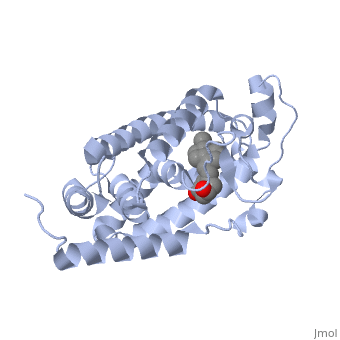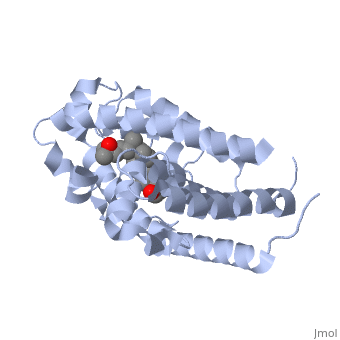
'''Vitamin D receptor''' (<scene name='56/562378/Vit_d_receptor_3m7r/3'>VDR</scene>) is a transcription factor. Upon binding to vitamin D, VDR forms a heterodimer with retinoid-X receptor and binds to hormone response receptors on DNA causing gene expression. The <scene name='56/562378/Vit_d_receptor_ligand/1'>vitamin D hormone</scene> (in green) binds to receptors in its target cells, controlling the synthesis of many different proteins involved in calcium transport and utilization. VDR contains two domains: a <scene name='56/562378/Lbd/1'>ligand binding domain (LBD)</scene> that binds to the hormone (grey) and <scene name='56/562378/Dbd/2'>DNA-binding domain (DBD)</scene> that binds to DNA. (Green and blue are two same VDR structures). It pairs up with a similar protein, 9-cis retinoic acid receptor (RXR), and together they bind to the DNA, activating synthesis in some cases and repressing it in others. | '''Vitamin D receptor''' (<scene name='56/562378/Vit_d_receptor_3m7r/3'>VDR</scene>) is a transcription factor. Upon binding to vitamin D, VDR forms a heterodimer with retinoid-X receptor and binds to hormone response receptors on DNA causing gene expression. The <scene name='56/562378/Vit_d_receptor_ligand/1'>vitamin D hormone</scene> (in green) binds to receptors in its target cells, controlling the synthesis of many different proteins involved in calcium transport and utilization. VDR contains two domains: a <scene name='56/562378/Lbd/1'>ligand binding domain (LBD)</scene> that binds to the hormone (grey) and <scene name='56/562378/Dbd/2'>DNA-binding domain (DBD)</scene> that binds to DNA. (Green and blue are two same VDR structures). It pairs up with a similar protein, 9-cis retinoic acid receptor (RXR), and together they bind to the DNA, activating synthesis in some cases and repressing it in others. | ||
| + | <!-- | ||
===Crystal Structure of the nuclear receptor for Vitamnin D complexed to Vitamin D=== | ===Crystal Structure of the nuclear receptor for Vitamnin D complexed to Vitamin D=== | ||
| Line 8: | Line 9: | ||
{{ABSTRACT_PUBMED_10678179}} | {{ABSTRACT_PUBMED_10678179}} | ||
| - | + | --> | |
==Disease== | ==Disease== | ||
[[http://www.uniprot.org/uniprot/VDR_HUMAN VDR_HUMAN]] Defects in VDR are the cause of rickets vitamin D-dependent type 2A (VDDR2A) [MIM:[http://omim.org/entry/277440 277440]]. A disorder of vitamin D metabolism resulting in severe rickets, hypocalcemia and secondary hyperparathyroidism. Most patients have total alopecia in addition to rickets.<ref>PMID:2849209</ref><ref>PMID:8381803</ref><ref>PMID:1652893</ref><ref>PMID:2177843</ref><ref>PMID:8106618</ref><ref>PMID:8392085</ref><ref>PMID:7828346</ref><ref>PMID:8675579</ref><ref>PMID:8961271</ref><ref>PMID:9005998</ref> | [[http://www.uniprot.org/uniprot/VDR_HUMAN VDR_HUMAN]] Defects in VDR are the cause of rickets vitamin D-dependent type 2A (VDDR2A) [MIM:[http://omim.org/entry/277440 277440]]. A disorder of vitamin D metabolism resulting in severe rickets, hypocalcemia and secondary hyperparathyroidism. Most patients have total alopecia in addition to rickets.<ref>PMID:2849209</ref><ref>PMID:8381803</ref><ref>PMID:1652893</ref><ref>PMID:2177843</ref><ref>PMID:8106618</ref><ref>PMID:8392085</ref><ref>PMID:7828346</ref><ref>PMID:8675579</ref><ref>PMID:8961271</ref><ref>PMID:9005998</ref> | ||
| Line 20: | Line 21: | ||
==Mutation== | ==Mutation== | ||
| - | <StructureSection load='VDRmutation1.pdb' size='350' side='right' caption='Mutation of Vitamin D Receptor' scene=''>In the article, "Phosphorylation of the Human Vitamin D receptor by Protein Kinase C" by Hsieh, J. et al, they presented their research on the mutation of serine to glycine and aspartic acid. They mentioned that amino acids like serine and threonine kinase plays a crucial role in signal transduction pathways drawn out by variety of growth factors, hormones, and neurotransmitters. When <scene name='56/562378/Serine_final/1'>serine</scene> is mutated it is replaced with a <scene name='56/562378/Glycine_final/1'>glycine</scene> which results in an inhibition of transcriptional activation. When transcription is inhibited it results in p53 accumulation, which activates and promotes p53 translocation into mitochondria leading to apoptosis. Transcription inhibition is useful in cancer patients and so can be used as treatment option. These are the outcomes of the mutation, with the research still in the process to find the potential cure for tumors. | + | <!-- <StructureSection load='VDRmutation1.pdb' size='350' side='right' caption='Mutation of Vitamin D Receptor' scene=''> -->In the article, "Phosphorylation of the Human Vitamin D receptor by Protein Kinase C" by Hsieh, J. et al, they presented their research on the mutation of serine to glycine and aspartic acid. They mentioned that amino acids like serine and threonine kinase plays a crucial role in signal transduction pathways drawn out by variety of growth factors, hormones, and neurotransmitters. When <scene name='56/562378/Serine_final/1'>serine</scene> is mutated it is replaced with a <scene name='56/562378/Glycine_final/1'>glycine</scene> which results in an inhibition of transcriptional activation. When transcription is inhibited it results in p53 accumulation, which activates and promotes p53 translocation into mitochondria leading to apoptosis. Transcription inhibition is useful in cancer patients and so can be used as treatment option. These are the outcomes of the mutation, with the research still in the process to find the potential cure for tumors. |
Revision as of 09:24, 4 February 2014
| |||||||||||
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Jaime Prilusky, Isita Amin