We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 930
From Proteopedia
(Difference between revisions)
| Line 8: | Line 8: | ||
[[Image:Actin myosin anim.gif|300px|left|thumb| The movement of myosin motor domain on actin filament, [[1cnt]]]] | [[Image:Actin myosin anim.gif|300px|left|thumb| The movement of myosin motor domain on actin filament, [[1cnt]]]] | ||
| - | Muscle contraction is achieved by the sliding of myosin filament (thick filament) | + | In the striated muscle the actin and myosin proteins form ordered basic units called sarcomeres. Muscle contraction is achieved by the mechanical sliding of myosin filament (thick filament) along the actin filament (thin filament). The major constituent of the myosin filament is myosin, a motor protein responsible for converting chemical energy to mechanical movement. In the presence of Ca2+ and Mg2+, myosin is able to cyclically bind ATP and hydrolyse it to ADP + Pi, triggering subsequent myosin-actin detachment, reattachment and power stroke, so called contractile reaction (Fig.1). |
| - | + | ||
| Line 24: | Line 23: | ||
| - | ==Myosin head S1== | + | ==Introduction of the Myosin head S1 == |
<StructureSection> | <StructureSection> | ||
<StructureSection load='1b7t.pdb' size='350' frame='true' side='right' caption='Myosin subfragment 1' scene='57/579700/Whole_structure/3' > | <StructureSection load='1b7t.pdb' size='350' frame='true' side='right' caption='Myosin subfragment 1' scene='57/579700/Whole_structure/3' > | ||
<StructureSection load='1B7T' size='350' frame='true' align='right' caption='Insert caption here' scene='57/579700/Whole_structure/3'> | <StructureSection load='1B7T' size='350' frame='true' align='right' caption='Insert caption here' scene='57/579700/Whole_structure/3'> | ||
| - | The myosin head comprises of a motor domain (DM) and a lever arm. fig!. The MD consists of 4 subdomains: the converter, the N-terminal subdomain, and upper and lower 50-kDa subdomains. They are linked together by 3 single-stranded joints termed the switch II, the relay, and SH1 helix. Conformational changes in these flexible joints coordinate rearrangements of the MD subdomains enabling the acto-myosin contractile cycle. The relatively small rearrangements of the MD domains cause large movement of the lever arm and thus enables the power stroke. The MD undergoes many conformational changes as it traduces ATP hydrolysis to mechanical work. The different conformational states of myosin are termed strong or weak actin-binding states. | ||
| + | Myosin is a large asymmetric molecule with a MW of about 500,000 kDa. It consist of a long tail and two globular head domains termed myosin subfragment 1 (S1), one neck subfragment 2 (S2) and a light meromyosin tail (LMM) (reference 1). Myosin S1 unit comprises of a motor domain (MD) and a lever arm (Fig.2). By 2000 the structures of three scallop myosin S1 isoforms have been determined (reference 2,3) , which are: | ||
| + | • S1 nucleotide-free state corresponding to the rigor state of myosin, actin complex (PDB link 1DFK) | ||
| + | • S1 Mg-ADP.VO4 state corresponding to the transition state (1kk8) | ||
| + | • S1 Mg-ADP state corresponding to the myosin detached state (1b7t) | ||
| - | ==Nucleotide binding pocket: ADP + | + | By comparing the available crystal structures of different myosin S1 unit isoforms, it enables us to understand the conformational changes within the motor domain depending on the nucleotide content in the active site. |
| + | Here mainly the structure and function of MD relevant in the S1 Mg-ADP (pre power stroke) state will be discussed. | ||
| + | |||
| + | |||
| + | ===The MD subdomains=== | ||
| + | |||
| + | (Fig 3) | ||
| + | |||
| + | https://www.uic.edu/classes/phyb/phyb516/BaranyUpdate4/Myosin/Myosin.html | ||
| + | |||
| + | The MD of S1 unit is most frequently described as consisting of 4 subdomains: the converter, the N-terminal subdomain, and upper and lower 50-kDa subdomains (ref 4). They are linked together by 3 single-stranded joints termed the switch II (residue IIe-461 to Asn-470), the relay (residues Asn-489 to ASP-519), and SH1 helix (Cys-693 to Phe-707). | ||
| + | • Of the MD subdomains the converter has the greatest positional change during contractile cycle. Connection of the converter and the lever arm allows relatively small changes in the converter to be greatly amplified in the lever arm fig? | ||
| + | • Upper 50-kDa subdomain and N-terminal subdomain form the nucleotide binding pocket | ||
| + | • Lower 50-kDa subdomain and converter form the interface where actin can bind | ||
| + | |||
| + | Conformational changes in the flexible joints coordinate rearrangements of these four MD subdomains enabling the transition between different myosin S1 unit states within the actomyosin contractile cycle. During the actomyosin cycle the MD undergoes many conformational changes as it traduces ATP hydrolysis to mechanical work. The different conformational states of myosin are termed strong or weak actin-binding states. | ||
| + | |||
| + | |||
| + | |||
| + | ==Nucleotide binding pocket: ADP + Mg2+== | ||
| + | |||
| + | The nucleotide-binding pocket is located at the interface of the 50 kDa upper subdomain and the N-terminal subdomain, which is opposite a deep cleft that bisects the actin-binding domain (the domain picture).This part of protein involves an arrangement of a secondary structure mainly around the parallel 7-stranded β-sheet (reference 1). Loops extending from b-strands interact with the adenine nucleotide. | ||
| + | |||
| + | ADP forms hydrogen bonds with the amino acide side chain around it (Scene), Mg2+ coordinates with side chains Thr183, Ser 241 of heavy chain, O1B and O3B from ADP and three water molecules (Scene) as well. The hydrogen bonds forming between ADP and side chains together with Mg2+ keeps ADP in the nucleotide-binding pocket. | ||
| + | |||
| + | In the contractile cycle, ATP binding causes a conformational change, which detaches the myosin S1 unit from actin. Then the active site closes, and ATP is hydrolysed to Pi and ADP, leading to the subsequent reattachment of the S1 with the actin. The conformational changes of the acting-binding pocket and the opening and closing of the nucleotide-binding pocket cause the strong and weak acting binding states of myosin, allowing muscle contraction. | ||
| - | kkk | ||
==Role of the subdomains and joints in the mechanism of the contractile cycle== | ==Role of the subdomains and joints in the mechanism of the contractile cycle== | ||
| + | |||
The MD has different conformational states in each step of the contractile cycle. The conformation of the MD in each state depends on which nucleotide is bound to the active site (if any). In each structural state the conformation of the MD changes relatively little, but these changes are enough to cause a substantial difference in the position of the lever arm. | The MD has different conformational states in each step of the contractile cycle. The conformation of the MD in each state depends on which nucleotide is bound to the active site (if any). In each structural state the conformation of the MD changes relatively little, but these changes are enough to cause a substantial difference in the position of the lever arm. | ||
Revision as of 07:45, 16 May 2014
| This Sandbox is Reserved from 01/04/2014, through 30/06/2014 for use in the course "510042. Protein structure, function and folding" taught by Prof Adrian Goldman, Tommi Kajander, Taru Meri, Konstantin Kogan and Juho Kellosalo at the University of Helsinki. This reservation includes Sandbox Reserved 923 through Sandbox Reserved 947. |
To get started:
More help: Help:Editing |
Contents |
Scallop myosin head in its pre power stroke state
Introduction
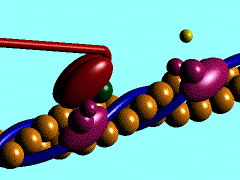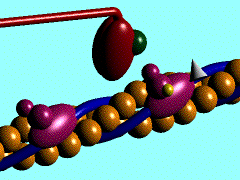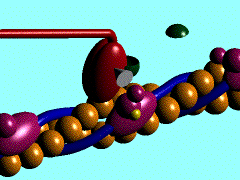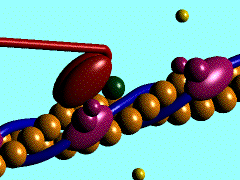
The movement of myosin motor domain on actin filament, 1cnt
In the striated muscle the actin and myosin proteins form ordered basic units called sarcomeres. Muscle contraction is achieved by the mechanical sliding of myosin filament (thick filament) along the actin filament (thin filament). The major constituent of the myosin filament is myosin, a motor protein responsible for converting chemical energy to mechanical movement. In the presence of Ca2+ and Mg2+, myosin is able to cyclically bind ATP and hydrolyse it to ADP + Pi, triggering subsequent myosin-actin detachment, reattachment and power stroke, so called contractile reaction (Fig.1).
.
Introduction of the Myosin head S1
| |||||||||||
