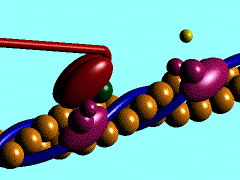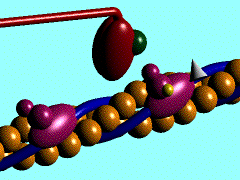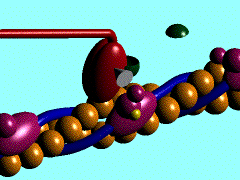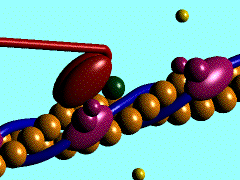
Myosin is a large asymmetric molecule with a MW of about 500,000 kDa. It consist of a long tail and two globular head domains termed myosin subfragment 1 (S1), one neck subfragment 2 (S2) and a light meromyosin tail (LMM) (reference 1). Myosin S1 unit comprises of a motor domain (MD) and a lever arm (Fig.2). By 2000 the structures of three scallop myosin S1 isoforms have been determined (reference 2,3) , which are:
• S1 nucleotide-free state corresponding to the rigor state of myosin, actin complex (PDB link 1DFK)
• S1 Mg-ADP.VO4 state corresponding to the transition state (1kk8)
• S1 Mg-ADP state corresponding to the myosin detached state (1b7t)
By comparing the available crystal structures of different myosin S1 unit isoforms, it enables us to understand the conformational changes within the motor domain depending on the nucleotide content in the active site.
Here mainly the structure and function of MD relevant in the S1 Mg-ADP (pre power stroke) state will be discussed.
The MD subdomains
(Fig 3)
https://www.uic.edu/classes/phyb/phyb516/BaranyUpdate4/Myosin/Myosin.html
The MD of S1 unit is most frequently described as consisting of 4 subdomains: the converter, the N-terminal subdomain, and upper and lower 50-kDa subdomains (ref 4). They are linked together by 3 single-stranded joints termed the switch II (residue IIe-461 to Asn-470), the relay (residues Asn-489 to ASP-519), and SH1 helix (Cys-693 to Phe-707).
• Of the MD subdomains the converter has the greatest positional change during contractile cycle. Connection of the converter and the lever arm allows relatively small changes in the converter to be greatly amplified in the lever arm fig?
• Upper 50-kDa subdomain and N-terminal subdomain form the nucleotide binding pocket
• Lower 50-kDa subdomain and converter form the interface where actin can bind
Conformational changes in the flexible joints coordinate rearrangements of these four MD subdomains enabling the transition between different myosin S1 unit states within the actomyosin contractile cycle. During the actomyosin cycle the MD undergoes many conformational changes as it traduces ATP hydrolysis to mechanical work. The different conformational states of myosin are termed strong or weak actin-binding states.
Nucleotide binding pocket: ADP + Mg2+
The nucleotide-binding pocket is located at the interface of the 50 kDa upper subdomain and the N-terminal subdomain, which is opposite a deep cleft that bisects the actin-binding domain (the domain picture).This part of protein involves an arrangement of a secondary structure mainly around the parallel 7-stranded β-sheet (reference 1). Loops extending from b-strands interact with the adenine nucleotide.
ADP forms hydrogen bonds with the amino acide side chain around it (Scene), Mg2+ coordinates with side chains Thr183, Ser 241 of heavy chain, O1B and O3B from ADP and three water molecules (Scene) as well. The hydrogen bonds forming between ADP and side chains together with Mg2+ keeps ADP in the nucleotide-binding pocket.
In the contractile cycle, ATP binding causes a conformational change, which detaches the myosin S1 unit from actin. Then the active site closes, and ATP is hydrolysed to Pi and ADP, leading to the subsequent reattachment of the S1 with the actin. The conformational changes of the acting-binding pocket and the opening and closing of the nucleotide-binding pocket cause the strong and weak acting binding states of myosin, allowing muscle contraction.
Role of the subdomains and joints in the mechanism of the contractile cycle
The MD has different conformational states in each step of the contractile cycle. The conformation of the MD in each state depends on which nucleotide is bound to the active site (if any). In each structural state the conformation of the MD changes relatively little, but these changes are enough to cause a substantial difference in the position of the lever arm.
The 50-kDa upper and lower subdomains as well as the converter control the motor function of the myosin head by rotating around the N-terminal subdomain. The rotations depend on the conformational changes of the 3 joints; switch II, SH1 helix region, and relay. The joints work together in the transition between the different conformational states of MD to control the overall organization of the myosin head. They also allow communication between the nucleotide-bonding pocket, acting-binding interface and the lever arm.
Switch II, a catalytic loop of the nucleotide-binding pocket, moves in and out of the nucleotide-binding pocket during enzymatic activity. It is responsible for the unwinding of SH1 helix, along with the conformational changes caused by nucleotide binding. Upon unwinding helix SH1 uncouples the converter/lever module from the MD. Movement of the converter is controlled by the relay joint. The converter/relay module attains different conformations changing the position of the lever arm and thus giving rise to the different states of the actomyosin cycle.
Taking part in the organization of the different conformations of the contractile cycle is also the so called switch I, which is a second catalytic loop of the nucleotide-binding pocket. Switch II forms a specific salt bridge and hydrogen bond interactions with switch I that stabilize the pre-power stroke state.
In the pre-power stroke conformation of the MD, switch II interacts with the nucleotide-binding pocket and forms the stabilizing hydrogen bond interactions and a salt bridge with switch I. fig? Rotation of the 50-kDa upper subdomain away from the N-terminal subdomain pulls switches I and II apart breaking the protein- nucleotide interactions between switch I and ADP, as well as changing the conformation of switch II. These changes result in closing of the actin-binding site and opening of the nucleotide-binding pocket, leading to MgADP release. At the same timeSH1 helix is unwound and the lever arm is able to change its position enabling sliding of myosin through the actin filament.
Conclusions
Mg
Mg and its coordination with other atoms
ADP
Section 3
This is the structure for to show. sdfbbsjfjsbfskjbf sjdfnjldns. IJI RR.