We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Gauri Misra/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 2: | Line 2: | ||
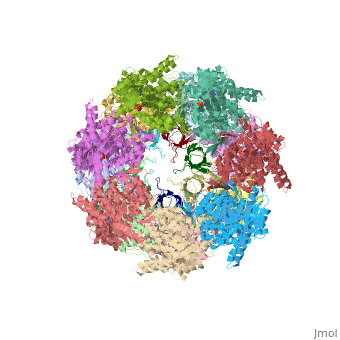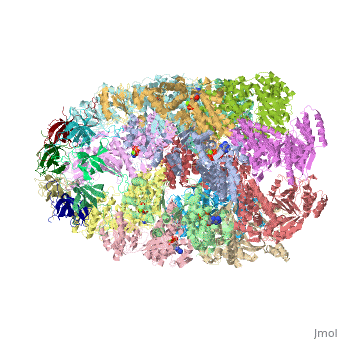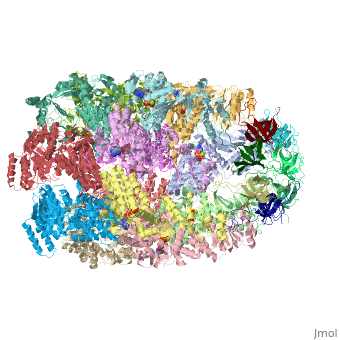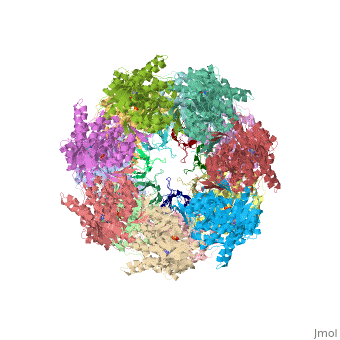
<StructureSection load='1aon' size='340' side='right' caption='The crystal structure of the asymmetric GroEL-GroES-(ADP)7 chaperonin complex' scene=''> | <StructureSection load='1aon' size='340' side='right' caption='The crystal structure of the asymmetric GroEL-GroES-(ADP)7 chaperonin complex' scene=''> | ||
Chaperones are proteins that are involved in the non-covalent folding and unfolding of other macromolecules. It exists both in prokaryotes and eukaryotes. Some chaperones are constitutively expressed in the system however, there are other chaperones that are expressed only in response to an external stimulus or stress such as heat and therefore they are referred to as ''heat shock proteins''. | Chaperones are proteins that are involved in the non-covalent folding and unfolding of other macromolecules. It exists both in prokaryotes and eukaryotes. Some chaperones are constitutively expressed in the system however, there are other chaperones that are expressed only in response to an external stimulus or stress such as heat and therefore they are referred to as ''heat shock proteins''. | ||
| - | <ref>PMID:21638687</ref>. | ||
| - | |||
== Function == | == Function == | ||
Chaperones bind to the newly synthesized proteins helping them acquire their properly folded 3D structure <ref>PMID: 3112578</ref> Besides, chaperones help in targeting the native proteins to their respective organelles <ref>PMID:3282178</ref><ref>DOI: 10.1002/iub.1272</ref> The first identified chaperones were the histone chaperones that are continously involved in histone metabolism thus regulating genome function, stability and identity<ref>doi: 10.1146/annurev-biochem-060713-035536</ref>. Many protozoan parasites such as ''Plasmodium falciparum'' requires these proteins for cytoprotection <ref>PMID: 14711509</ref> <ref>PMID: 19339102</ref>Chaperones actively participate in the maintenance of proteome integrity, and protein homeostasis (proteostasis) which requires a syncrhonization in various chaperones tuning the process <ref>DOI: 10.1146/annurev-biochem-060208-092442</ref>. | Chaperones bind to the newly synthesized proteins helping them acquire their properly folded 3D structure <ref>PMID: 3112578</ref> Besides, chaperones help in targeting the native proteins to their respective organelles <ref>PMID:3282178</ref><ref>DOI: 10.1002/iub.1272</ref> The first identified chaperones were the histone chaperones that are continously involved in histone metabolism thus regulating genome function, stability and identity<ref>doi: 10.1146/annurev-biochem-060713-035536</ref>. Many protozoan parasites such as ''Plasmodium falciparum'' requires these proteins for cytoprotection <ref>PMID: 14711509</ref> <ref>PMID: 19339102</ref>Chaperones actively participate in the maintenance of proteome integrity, and protein homeostasis (proteostasis) which requires a syncrhonization in various chaperones tuning the process <ref>DOI: 10.1146/annurev-biochem-060208-092442</ref>. | ||
== Disease == | == Disease == | ||
| - | + | Chaperones are instrumental in protein folding processes. Any alteration in this process leads to protein aggregation and formation of inclusion bodies. Protein misfolding results in various diseases as summarized in the following table: | |
| + | Table 1: Neurodegenerative diseases and protein deposits <ref>http://dx.doi.org/10.1155/2011/618127</ref> | ||
| + | Disease Inclusion Abnormal protein | ||
| + | Alzheimer disease Extracellular neuritic plaque Aβ peptide | ||
| + | Cytosolic neurofibrillatory tangles Tau | ||
| + | Parkinson disease Lewy bodies α-synuclein | ||
| + | Familial amyotrophic lateral sclerosis Intracellular inclusions SOD1 | ||
| + | Huntington disease Nuclear, cytosolic inclusion Huntingtin | ||
| + | Spinocerebellar ataxia 1, 2, 3 Nuclear inclusions Ataxin 1, 2, 3 | ||
| + | Spinobulbar muscular atrophy Nuclear inclusions Androgen receptors | ||
== Relevance == | == Relevance == | ||
Revision as of 07:24, 10 June 2014
'Chaperones'
| |||||||||||
References
- ↑ Ellis J. Proteins as molecular chaperones. Nature. 1987 Jul 30-Aug 5;328(6129):378-9. PMID:3112578 doi:http://dx.doi.org/10.1038/328378a0
- ↑ Deshaies RJ, Koch BD, Werner-Washburne M, Craig EA, Schekman R. A subfamily of stress proteins facilitates translocation of secretory and mitochondrial precursor polypeptides. Nature. 1988 Apr 28;332(6167):800-5. PMID:3282178 doi:http://dx.doi.org/10.1038/332800a0
- ↑ Halperin L, Jung J, Michalak M. The many functions of the endoplasmic reticulum chaperones and folding enzymes. IUBMB Life. 2014 May 19. doi: 10.1002/iub.1272. PMID:24839203 doi:http://dx.doi.org/10.1002/iub.1272
- ↑ Gurard-Levin ZA, Quivy JP, Almouzni G. Histone chaperones: assisting histone traffic and nucleosome dynamics. Annu Rev Biochem. 2014 Jun 2;83:487-517. doi:, 10.1146/annurev-biochem-060713-035536. PMID:24905786 doi:http://dx.doi.org/10.1146/annurev-biochem-060713-035536
- ↑ Matambo TS, Odunuga OO, Boshoff A, Blatch GL. Overproduction, purification, and characterization of the Plasmodium falciparum heat shock protein 70. Protein Expr Purif. 2004 Feb;33(2):214-22. PMID:14711509
- ↑ Misra G, Ramachandran R. Hsp70-1 from Plasmodium falciparum: protein stability, domain analysis and chaperone activity. Biophys Chem. 2009 Jun;142(1-3):55-64. doi: 10.1016/j.bpc.2009.03.006. Epub 2009 , Mar 16. PMID:19339102 doi:http://dx.doi.org/10.1016/j.bpc.2009.03.006
- ↑ Kim YE, Hipp MS, Bracher A, Hayer-Hartl M, Hartl FU. Molecular chaperone functions in protein folding and proteostasis. Annu Rev Biochem. 2013;82:323-55. doi: 10.1146/annurev-biochem-060208-092442. PMID:23746257 doi:http://dx.doi.org/10.1146/annurev-biochem-060208-092442
- ↑ http://dx.doi.org/10.1155/2011/618127