We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Chaperones
From Proteopedia
(Difference between revisions)
| Line 12: | Line 12: | ||
== Structural highlights == | == Structural highlights == | ||
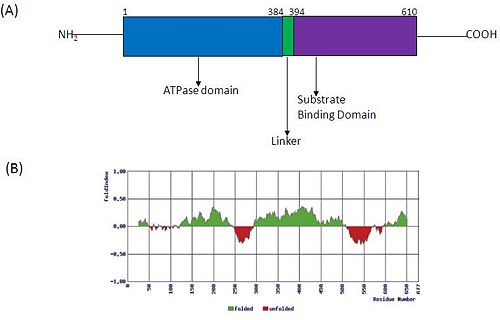
| - | Structurally, hsp70 have a N-terminal <scene name='59/591341/Nbd_hsp70/7'>ATPase domain</scene> followed by a <scene name='59/591341/Sbd_of_dnak1/2'>substrate binding domain</scene> with elongated C-terminal. These domains allosterically regulate the hsp70 functioning. In the 2D figure given below, panel A shows the structural organization of Hsp 70 indicating its various domains. ATP binding and hydrolysis regulates the affinity for substrate proteins which thereafter enhances ATP hydrolysis. Panel B predicts the various folded (green) and unfolded (red)regions in Hsp70. | + | Structurally, hsp70 have a N-terminal <scene name='59/591341/Nbd_hsp70/7'>ATPase domain</scene> followed by a <scene name='59/591341/Sbd_of_dnak1/2'>substrate binding domain</scene> with elongated C-terminal. These domains allosterically regulate the hsp70 functioning. In the 2D figure given below, panel A shows the structural organization of Hsp 70 indicating its various domains. ATP binding and hydrolysis regulates the affinity for substrate proteins which thereafter enhances ATP hydrolysis. Panel B predicts the various folded (green) and unfolded (red) regions in Hsp70. |
[[Image:HSP_70.JPG|left|500px|thumb|Structural organization of Hsp70]] | [[Image:HSP_70.JPG|left|500px|thumb|Structural organization of Hsp70]] | ||
</StructureSection> | </StructureSection> | ||
Revision as of 14:02, 19 June 2014
| |||||||||||
References
- ↑ Kim YE, Hipp MS, Bracher A, Hayer-Hartl M, Hartl FU. Molecular chaperone functions in protein folding and proteostasis. Annu Rev Biochem. 2013;82:323-55. doi: 10.1146/annurev-biochem-060208-092442. PMID:23746257 doi:http://dx.doi.org/10.1146/annurev-biochem-060208-092442
- ↑ Ellis J. Proteins as molecular chaperones. Nature. 1987 Jul 30-Aug 5;328(6129):378-9. PMID:3112578 doi:http://dx.doi.org/10.1038/328378a0
- ↑ Deshaies RJ, Koch BD, Werner-Washburne M, Craig EA, Schekman R. A subfamily of stress proteins facilitates translocation of secretory and mitochondrial precursor polypeptides. Nature. 1988 Apr 28;332(6167):800-5. PMID:3282178 doi:http://dx.doi.org/10.1038/332800a0
- ↑ Halperin L, Jung J, Michalak M. The many functions of the endoplasmic reticulum chaperones and folding enzymes. IUBMB Life. 2014 May 19. doi: 10.1002/iub.1272. PMID:24839203 doi:http://dx.doi.org/10.1002/iub.1272
- ↑ Gurard-Levin ZA, Quivy JP, Almouzni G. Histone chaperones: assisting histone traffic and nucleosome dynamics. Annu Rev Biochem. 2014 Jun 2;83:487-517. doi:, 10.1146/annurev-biochem-060713-035536. PMID:24905786 doi:http://dx.doi.org/10.1146/annurev-biochem-060713-035536
- ↑ Matambo TS, Odunuga OO, Boshoff A, Blatch GL. Overproduction, purification, and characterization of the Plasmodium falciparum heat shock protein 70. Protein Expr Purif. 2004 Feb;33(2):214-22. PMID:14711509
- ↑ Misra G, Ramachandran R. Hsp70-1 from Plasmodium falciparum: protein stability, domain analysis and chaperone activity. Biophys Chem. 2009 Jun;142(1-3):55-64. doi: 10.1016/j.bpc.2009.03.006. Epub 2009 , Mar 16. PMID:19339102 doi:http://dx.doi.org/10.1016/j.bpc.2009.03.006
- ↑ Kim YE, Hipp MS, Bracher A, Hayer-Hartl M, Hartl FU. Molecular chaperone functions in protein folding and proteostasis. Annu Rev Biochem. 2013;82:323-55. doi: 10.1146/annurev-biochem-060208-092442. PMID:23746257 doi:http://dx.doi.org/10.1146/annurev-biochem-060208-092442
- ↑ Meriin AB, Sherman MY. Role of molecular chaperones in neurodegenerative disorders. Int J Hyperthermia. 2005 Aug;21(5):403-19. PMID:16048838 doi:http://dx.doi.org/10.1080/02656730500041871
- ↑ Winklhofer KF, Tatzelt J. The role of chaperones in Parkinson's disease and prion diseases. Handb Exp Pharmacol. 2006;(172):221-58. PMID:16610362
- ↑ Jain MR, Ge WW, Elkabes S, Li H. Amyotrophic lateral sclerosis: Protein chaperone dysfunction revealed by proteomic studies of animal models. Proteomics Clin Appl. 2008 May 1;2(5):670-684. PMID:19578526 doi:http://dx.doi.org/10.1002/prca.200780023
- ↑ Qi L, Zhang XD. Role of chaperone-mediated autophagy in degrading Huntington's disease-associated huntingtin protein. Acta Biochim Biophys Sin (Shanghai). 2014 Feb;46(2):83-91. doi:, 10.1093/abbs/gmt133. Epub 2013 Dec 8. PMID:24323530 doi:http://dx.doi.org/10.1093/abbs/gmt133
- ↑ Paulson H. Machado-Joseph disease/spinocerebellar ataxia type 3. Handb Clin Neurol. 2012;103:437-49. doi: 10.1016/B978-0-444-51892-7.00027-9. PMID:21827905 doi:http://dx.doi.org/10.1016/B978-0-444-51892-7.00027-9
- ↑ Bailey CK, Andriola IF, Kampinga HH, Merry DE. Molecular chaperones enhance the degradation of expanded polyglutamine repeat androgen receptor in a cellular model of spinal and bulbar muscular atrophy. Hum Mol Genet. 2002 Mar 1;11(5):515-23. doi: 10.1093/hmg/11.5.515. PMID:11875046 doi:http://dx.doi.org/10.1093/hmg/11.5.515
- ↑ Chaudhuri TK, Paul S. Protein-misfolding diseases and chaperone-based therapeutic approaches. FEBS J. 2006 Apr;273(7):1331-49. PMID:16689923 doi:http://dx.doi.org/10.1111/j.1742-4658.2006.05181.x
- ↑ Ebrahimi-Fakhari D, Saidi LJ, Wahlster L. Molecular chaperones and protein folding as therapeutic targets in Parkinson's disease and other synucleinopathies. Acta Neuropathol Commun. 2013 Dec 5;1(1):79. doi: 10.1186/2051-5960-1-79. PMID:24314025 doi:http://dx.doi.org/10.1186/2051-5960-1-79