Gag polyprotein
From Proteopedia
| Line 31: | Line 31: | ||
| - | The '''Gag polyprotein''' (Gag) is part of the basic infrastructure of retroviruses. The Gag is processed during maturation | + | The '''Gag polyprotein''' (Gag) is part of the basic infrastructure of retroviruses. The Gag is processed during maturation to '''matrix protein (MA)''', '''capsid protein (CA)''', '''spacer peptides (SP1, SP2)''', '''nucleocapsid protein (NC)''' and '''p6'''. For detailed discussion of HIV-1 Gag polyprotein see [[Hiv-1 gag]]. |
Gag of [http://en.wikipedia.org/wiki/Human_immunodeficiency_virus_type_1_(isolate_12) human immunodeficiency virus type 1] (HIV-1), is a primary protein involved in the packaging of two copies of the viral genome for capsid formation <ref>Coffin, J., S. Hughes, and H. Varmus, Retroviruses. 1997: Cold Spring Harbor Laboratory Press.</ref>. In the cytoplasm of the infected cell, Gag is translated and approximately 1500 copies of the immature HIV-1 Gag polyprotein [[1l6n]] come together to form an immature viral particle. After budding of the viral particle, viral proteases cleave the Gag protein into three structurally different products: the matrix, the capsid, and the nucleocapsid. The protealytic cleavage results in viral maturation and induces significant structural changes of the protein products. Following this proteolysis, the viral particle takes on the classic cone shape required for a new infection. | Gag of [http://en.wikipedia.org/wiki/Human_immunodeficiency_virus_type_1_(isolate_12) human immunodeficiency virus type 1] (HIV-1), is a primary protein involved in the packaging of two copies of the viral genome for capsid formation <ref>Coffin, J., S. Hughes, and H. Varmus, Retroviruses. 1997: Cold Spring Harbor Laboratory Press.</ref>. In the cytoplasm of the infected cell, Gag is translated and approximately 1500 copies of the immature HIV-1 Gag polyprotein [[1l6n]] come together to form an immature viral particle. After budding of the viral particle, viral proteases cleave the Gag protein into three structurally different products: the matrix, the capsid, and the nucleocapsid. The protealytic cleavage results in viral maturation and induces significant structural changes of the protein products. Following this proteolysis, the viral particle takes on the classic cone shape required for a new infection. | ||
Revision as of 09:33, 27 July 2014
The Gag polyprotein (Gag) is part of the basic infrastructure of retroviruses. The Gag is processed during maturation to matrix protein (MA), capsid protein (CA), spacer peptides (SP1, SP2), nucleocapsid protein (NC) and p6. For detailed discussion of HIV-1 Gag polyprotein see Hiv-1 gag.
Gag of human immunodeficiency virus type 1 (HIV-1), is a primary protein involved in the packaging of two copies of the viral genome for capsid formation [2]. In the cytoplasm of the infected cell, Gag is translated and approximately 1500 copies of the immature HIV-1 Gag polyprotein 1l6n come together to form an immature viral particle. After budding of the viral particle, viral proteases cleave the Gag protein into three structurally different products: the matrix, the capsid, and the nucleocapsid. The protealytic cleavage results in viral maturation and induces significant structural changes of the protein products. Following this proteolysis, the viral particle takes on the classic cone shape required for a new infection.
Capsid (CA) Domain
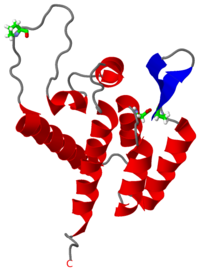
Overall, the mature CAN domain is very similar in structure to the corresponding domain of the immature Gag283 polyprotein [1]. The CAN protein contains 7 α-helices (helix 1-helix 7) that pack together to form a triangular shape, which helps facilitate the final complex formation for the capsid core particles. There are two significant structural differences between the immature and mature versions of the CAN domain: an N-terminal β-hairpin and a 2-Angstrom displacement of helix 6.
In the mature CAN protein, the N-terminal residues form an anti-parallel β-hairpin instead of the random coil that is observed when the same residues are compared in the immature Gag283 polyprotein. The NH2+ group of the N-terminus proline establishes a salt bridge with a nearby aspartic acid, which stabilizes the β-hairpin. This N-terminal β-hairpin is required for the final formation of the viral capsid, and many studies have shown through conservation and mutagenesis that this β-hairpin is responsible for the stabilization of the protein complexes involved in the capsid formation [3][4].
As a side effect of the β-hairpin formation, the helix 6 is displaced by approximately 2-Angstroms. This displacement, though structurally minor, causes significant biological changes in the protein. Most importantly, helix 6 interacts with the protein cyclophilin A (CypA)-binding site. CypA is a prolyl isomerase and chaperone protein involved in the infection process by aiding in unpacking the capsid [5][6][7].
Implications
HIV-1 viral particles need to form a capsid cone-like structure prior to infection of the host cell. The protealytic cleavage of the immature Gag283 polyprotein results in a capsid domain. This post-translational modification is essential to the formation of the core structure. Many studies have shown that the β-hairpin formed after maturation is essential for the capsid core particle formation [3][4]. As a result of the β-hairpin formation, the helix 6 is displaced causing an allosteric mechanism for CpyA binding. Overall, the maturation of Gag283 and formation of the mature CA protein is essential for core capsid particle creation and consequently final infection.
3D structures of Gag polyprotein
Updated on 27-July-2014
Gag polyprotein from HIV-1
2h3i – Gag residues 2-132 – HIV-1
2h3f, 2h3i, 1uph - Gag residues 2-132 – NMR
1l6n - Gag residues 1-283 – NMR
1gwp - Gag residues 132-283 – NMR
2jmg, 2nv3 - Gag residues 2-132 (mutant) – NMR
1u57 - Gag residues 1-48 – NMR
1esk - Gag residues 12-53 – NMR
1baj – Gag C terminal
2znf – Gag zinc fingerlike domain - NMR
2h3q, 2h3v, 2h3z - Gag residues 2-132 + phosphatidyl inositol bisphosphate - NMR
1mt7, 1mt8 – Gag MA-CA cleavage site + protease retropepsin (mutant)
1agb, 1agc, 1agd, 1age, 1agf – Gag peptide + β-2 microglobulin + B*0801
3p9g, 3p9h, 3obu, 3obx, 1m4p, 1m4q - Gag peptide + tumor susceptibility gene 101 protein N terminal
1fgl – Gag residues 81-105 + cyclophilin A
2xde - Gag residues 1-146 + inhibitor
4e91, 4e92 - Gag residues 133-278 + inhibitor
2x2d - Gag residues 133-278 + peptidyl-prolyl cis-trans isomerase A
2lf4 - Gag residues 133-363 (mutant)
2xt1 – Gag C terminal + camelid VHH
1sje, 1sjh – Gag peptide + HLA-DR1
1kj4, 1kj7, 1kjf, 1kjg - Gag peptide + POL polyprotein
Gag polyprotein from HIV-2
2wlv - Gag residues 99-242 – HIV-2
2ec7 – Gag – NMR
Gag polyprotein from Rous sarcoma virus
3g0v, 3g1g, 3g21, 3g1i - Gag C terminal – Rous sarcoma virus
3g26, 3g28, 3g29 - Gag C terminal (mutant)
1p7n – Gag N terminal
1em9 - Gag N terminal – NMR
1eoq - Gag C terminal – NMR
1a6s – Gag M domain (mutant) - NMR
Gag polyprotein from Simian immunodeficiency virus
1ecw, 1ed1 - Gag
2xs1 - Gag peptide + programmed cell death 6-interacting protein
Gag polyprotein from Moloney murine leukemia virus
1u7k, 3bp9 - Gag residues 215-345
1u6p – Gag fragment + DNA – Moloney murine leukemia virus
Gag polyprotein from equine infectious anemia virus
1hek – Gag
Gag polyprotein from Mason-Pfizer monkey virus
1cl4 - Gag residues 49-80 (mutant) – NMR
Gag polyprotein from human spumaretrovirus
4jnh - Gag N terminal
Additional Resources
For additional information, see: Human Immunodeficiency Virus
Reference
- ↑ 1.0 1.1 Tang C, Ndassa Y, Summers MF. Structure of the N-terminal 283-residue fragment of the immature HIV-1 Gag polyprotein. Nat Struct Biol. 2002 Jul;9(7):537-43. PMID:12032547 doi:10.1038/nsb806
- ↑ Coffin, J., S. Hughes, and H. Varmus, Retroviruses. 1997: Cold Spring Harbor Laboratory Press.
- ↑ 3.0 3.1 Gitti RK, Lee BM, Walker J, Summers MF, Yoo S, Sundquist WI. Structure of the amino-terminal core domain of the HIV-1 capsid protein. Science. 1996 Jul 12;273(5272):231-5. PMID:8662505
- ↑ 4.0 4.1 von Schwedler UK, Stemmler TL, Klishko VY, Li S, Albertine KH, Davis DR, Sundquist WI. Proteolytic refolding of the HIV-1 capsid protein amino-terminus facilitates viral core assembly. EMBO J. 1998 Mar 16;17(6):1555-68. PMID:9501077 doi:10.1093/emboj/17.6.1555
- ↑ Braaten D, Franke EK, Luban J. Cyclophilin A is required for an early step in the life cycle of human immunodeficiency virus type 1 before the initiation of reverse transcription. J Virol. 1996 Jun;70(6):3551-60. PMID:8648689
- ↑ Thali M, Bukovsky A, Kondo E, Rosenwirth B, Walsh CT, Sodroski J, Gottlinger HG. Functional association of cyclophilin A with HIV-1 virions. Nature. 1994 Nov 24;372(6504):363-5. PMID:7969495 doi:http://dx.doi.org/10.1038/372363a0
- ↑ Ackerson B, Rey O, Canon J, Krogstad P. Cells with high cyclophilin A content support replication of human immunodeficiency virus type 1 Gag mutants with decreased ability to incorporate cyclophilin A. J Virol. 1998 Jan;72(1):303-8. PMID:9420228
Team from University of Missouri, Columbia, MO
- Students: Zheng Wang, Allison Tegge, Xin Deng
- Advisors: Jianlin Cheng, PhD, Department of Computer Science, Informatics Institute, the Life Science Center, Interdisciplinary Plant Group, University of Missouri, Columbia
- Mentor: Chun Tang, PhD, Department of Biochemistry, University of Missouri, Columbia
NMR Equipment and the Authors
Created by Allison Tegge and David Canner