Acetylcholinesterase
From Proteopedia
| Line 54: | Line 54: | ||
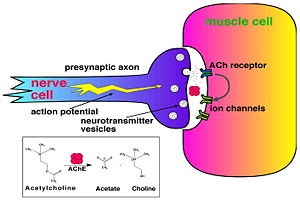
As was mentioned above, AChE hydrolysizes the neurotransmitter <scene name='2wfz/Al/2'>ACh</scene>, producing <scene name='2wfz/Al/3'>choline and an acetate</scene> group. <scene name='2wfz/Al/2'>ACh</scene> directly binds catalytic <scene name='2wfz/Al/4'>Ser200</scene> (via its nucleophilic Oγ atom). <scene name='2wfz/Al/5'>Soman</scene>, [http://en.wikipedia.org/wiki/Soman O-(1,2,2-trimethylpropyl) methylphosphonofluoridate] (<font color='violet'><b>fluorine atom is colored violet</b></font> and <font color='darkmagenta'><b>phosphorus atom is colored darkmagenta</b></font>), is one of the most toxic OPs. Soman inhibits AChE by <scene name='2wfz/Al/6'>covalent binding</scene> to catalytic Ser200, <scene name='2wfz/Al/7'>mimicking ACh</scene>. This process <scene name='2wfz/Al/8'>(phosphonylation)</scene> implicates nucleophilic attack of the Ser200 nucleophilic Oγ atom on the phosphorus atom of soman, with concomitant departure of its fluoride atom. After that AChE catalyzes the <scene name='2wfz/Al/9'>dealkylation</scene> ("aging") of the soman or other OP. This causes irreversible inhibition of AChE, "aged" soman/AChE conjugate can not be reactivated. However, before “aging”, at the step of <scene name='2wfz/Al/8'>phosphonylation</scene>, AChE can be <scene name='2wfz/Al/11'>reactivated</scene> by nucleophiles, such as pralidoxime (2-PAM), resulting in <scene name='2wfz/Al/12'>cleavage</scene> of the phosphonyl adduct from Ser200 Oγ. | As was mentioned above, AChE hydrolysizes the neurotransmitter <scene name='2wfz/Al/2'>ACh</scene>, producing <scene name='2wfz/Al/3'>choline and an acetate</scene> group. <scene name='2wfz/Al/2'>ACh</scene> directly binds catalytic <scene name='2wfz/Al/4'>Ser200</scene> (via its nucleophilic Oγ atom). <scene name='2wfz/Al/5'>Soman</scene>, [http://en.wikipedia.org/wiki/Soman O-(1,2,2-trimethylpropyl) methylphosphonofluoridate] (<font color='violet'><b>fluorine atom is colored violet</b></font> and <font color='darkmagenta'><b>phosphorus atom is colored darkmagenta</b></font>), is one of the most toxic OPs. Soman inhibits AChE by <scene name='2wfz/Al/6'>covalent binding</scene> to catalytic Ser200, <scene name='2wfz/Al/7'>mimicking ACh</scene>. This process <scene name='2wfz/Al/8'>(phosphonylation)</scene> implicates nucleophilic attack of the Ser200 nucleophilic Oγ atom on the phosphorus atom of soman, with concomitant departure of its fluoride atom. After that AChE catalyzes the <scene name='2wfz/Al/9'>dealkylation</scene> ("aging") of the soman or other OP. This causes irreversible inhibition of AChE, "aged" soman/AChE conjugate can not be reactivated. However, before “aging”, at the step of <scene name='2wfz/Al/8'>phosphonylation</scene>, AChE can be <scene name='2wfz/Al/11'>reactivated</scene> by nucleophiles, such as pralidoxime (2-PAM), resulting in <scene name='2wfz/Al/12'>cleavage</scene> of the phosphonyl adduct from Ser200 Oγ. | ||
At the <scene name='2wfz/Ali/3'>active site of the nonaged soman/TcAChE conjugate</scene> ([[2wfz]]) the catalytic His440 forms hydrogen bonds with Ser200 Oγ and Glu327 Oε1 via its Nε2 and Nδ1 nitrogens, respectively. The O2 atom of soman is within hydrogen bonding distance of His440 Nε2. Soman O1 mimicks carbonyl oxygen of ACh. A water molecule 1001 interacting with soman O2 is represented as a <font color='red'><b>red ball</b></font>. The active site residues of the nonaged soman/TcAChE are colored <span style="color:yellow;background-color:black;font-weight:bold;">yellow</span>. The O2 atom of the <scene name='2wfz/Ali/4'>dealkylated (aged) soman</scene> ([[2wg0]]) forms a salt bridge with His440 Nε2. The active site residues of the aged soman/TcAChE are colored <span style="color:pink;background-color:black;font-weight:bold;">pink</span>. <scene name='2wfz/Ali/5'>Alignment</scene> of the structures of the nonaged ([[2wfz]]) and aged ([[2wg0]]) conjugates reveals a small, but important, change within the active site - the imidazole ring of His440 is tilted back to a native-like conformation after dealkylation. The water molecule 1001, which interacts with soman O2 in the nonaged crystal structure, is not within hydrogen bonding distance of O2 in the aged crystal structure. 2-PAM binds poorly to the nonaged phosphonylated enzyme (its electron density was not found) and binds in an <scene name='2wfz/Ali/7'>unfavorable and nonfunctional conformation</scene> after soman aging to ''Tc''AChE ([[2wg1]]) <ref name="Sanson">PMID:19642642</ref>. | At the <scene name='2wfz/Ali/3'>active site of the nonaged soman/TcAChE conjugate</scene> ([[2wfz]]) the catalytic His440 forms hydrogen bonds with Ser200 Oγ and Glu327 Oε1 via its Nε2 and Nδ1 nitrogens, respectively. The O2 atom of soman is within hydrogen bonding distance of His440 Nε2. Soman O1 mimicks carbonyl oxygen of ACh. A water molecule 1001 interacting with soman O2 is represented as a <font color='red'><b>red ball</b></font>. The active site residues of the nonaged soman/TcAChE are colored <span style="color:yellow;background-color:black;font-weight:bold;">yellow</span>. The O2 atom of the <scene name='2wfz/Ali/4'>dealkylated (aged) soman</scene> ([[2wg0]]) forms a salt bridge with His440 Nε2. The active site residues of the aged soman/TcAChE are colored <span style="color:pink;background-color:black;font-weight:bold;">pink</span>. <scene name='2wfz/Ali/5'>Alignment</scene> of the structures of the nonaged ([[2wfz]]) and aged ([[2wg0]]) conjugates reveals a small, but important, change within the active site - the imidazole ring of His440 is tilted back to a native-like conformation after dealkylation. The water molecule 1001, which interacts with soman O2 in the nonaged crystal structure, is not within hydrogen bonding distance of O2 in the aged crystal structure. 2-PAM binds poorly to the nonaged phosphonylated enzyme (its electron density was not found) and binds in an <scene name='2wfz/Ali/7'>unfavorable and nonfunctional conformation</scene> after soman aging to ''Tc''AChE ([[2wg1]]) <ref name="Sanson">PMID:19642642</ref>. | ||
| + | |||
| + | == Structural and functional characterization of the interaction of the photosensitizing probe methylene blue with ''Torpedo californica'' acetylcholinesterase <ref >doi 10.1002/pro.2101</ref>== | ||
| + | |||
| + | <hr/> | ||
| + | |||
| + | The photosensitizer, <scene name='Journal:Protein_Science:1/Cv/3'>methylene blue (MB)</scene> <font color='darkmagenta'><b>(colored in darkmagenta)</b></font>, generates singlet oxygen that irreversibly inhibits Torpedo californica acetylcholinesterase (''Tc''AChE). In the dark, it inhibits reversibly. | ||
| + | The ''Tc''AChE active site consists of two binding subsites. One of them is the '''"catalytic anionic site" (CAS)''', which involves the catalytic triad <scene name='Journal:Protein_Science:1/Cv/6'>Ser200, His440, and Glu327</scene> <span style="color:orange;background-color:black;font-weight:bold;">(colored in orange)</span> and the conserved residues <scene name='Journal:Protein_Science:1/Cv/8'>Trp84 and Phe330</scene> which also participate in ligand recognition. Another conserved residue <scene name='Journal:Protein_Science:1/Cv/9'>Trp279</scene> <span style="color:cyan;background-color:black;font-weight:bold;">(colored in cyan)</span> is situated at the second binding subsite, termed the '''"peripheral anionic site" (PAS)''', ~14 Å from CAS. <scene name='Journal:Protein_Science:1/Cv/10'>Thioflavin T</scene> ([[2j3q]]) is a good example of the '''PAS-binding''' AChE inhibitors. <scene name='Journal:Protein_Science:1/Cv/11'>Superposition</scene> of the structure of known '''CAS-binding''' inhibitor <font color='crimson'><b>edrophonium</b></font>/''Tc''AChE ([[2ack]]) on the <font color='magenta'><b>thioflavin T</b></font>/''Tc''AChE complex structure ([[2j3q]]) shows that these <scene name='Journal:Protein_Science:1/Cv/12'>ligands' positions do not overlap</scene><ref name="Ravelli">PMID:10089512</ref> <ref name="Sonoda">PMID:18512913</ref>. | ||
| + | MB is a noncompetitive inhibitor of ''Tc''AChE, competing with reversible inhibitors directed at both ‘‘anionic’’ subsites, but a single site is involved in inhibition. The crystal structure reveals a <scene name='Journal:Protein_Science:1/Cv1/2'>single MB stacked against Trp279 in the PAS</scene>, oriented down the gorge toward the CAS ([[2w9i]]); it is plausible that irreversible inhibition is associated with photooxidation of this residue and others within the active-site gorge. Superposition of the '''PAS regions''' of the <font color='darkmagenta'><b>MB</b></font>/''Tc''AChE ([[2w9i]]) and <font color='magenta'><b>thioflavin T</b></font>/''Tc''AChE ([[2j3q]]) complexes reveals <scene name='Journal:Protein_Science:1/Cv1/4'>similarity between positions of these ligands</scene>. As the conformation of ''Tc''AChE in the crystal structures of the two complexes is practically identical, only that of the <font color='darkmagenta'><b>MB</b></font>/''Tc''AChE structure ([[2w9i]]) is shown. The kinetic and spectroscopic data showing that inhibitors binding at the '''CAS''' can impede binding of MB are reconciled by docking studies showing that the <scene name='Journal:Protein_Science:1/Cv2/5'>conformation adopted by Phe330</scene>, midway down the gorge, in the MB/''Tc''AChE crystal structure, precludes simultaneous binding of a second MB at the CAS (<font color='blueviolet'><b>2nd MB is colored blueviolet</b></font>, <span style="color:orange;background-color:black;font-weight:bold;">Phe330 of the crystal structure is in orange</span> and <font color='indigo'><b>Phe330 of the modeled structure is in indigo</b></font>). Conversely, binding of ligands at the '''CAS''' dislodges MB from its preferred locus at the '''PAS'''. The data presented demonstrate that TcAChE is a valuable model for understanding the molecular basis of local photooxidative damage. | ||
For more details see<br /> | For more details see<br /> | ||
Revision as of 11:50, 2 October 2014
| |||||||||||
3D Structures of AChE
Updated on 02-October-2014
Acetylcholinesterase - AChE native
3lii, 4ey4 – hAChE - recombinant human
1ea5, 2ace – TcAChE – trigonal – Torpedo californica
2j3d – TcAChE – monoclinic
1w75 – TcAChE – orthorhombic
2vt6, 2vt7 – TcAChE – different dosage
1qid to 1qim - TcAChE synchrotron radiation damage
1j06, 1maa – mAChE - mouse
1qo9 – DmAChE - Drosophila melanogaster
1eea, 1c2b, 1c2o – AChE – Electric eel
AChE inhibitors (In Different Languages)
1eve AChE-Aricept complex, 1eve (Arabic), 1eve (Chinese), 1eve (Italian), 1eve (Russian), 1eve (Spanish), 1eve (Turkish)
1vot AChE-Huperzine A complex, 1vot (Chinese)
AChE active site inhibitors conjugating at the bottom of the active site gorge
2c4h – TcAChE + acetylthiocholine
2w9i – TcAChE + methylene blue
2wls – MosAChE + AMTS13
2vq6 – TcAChE + 2-PAM
2j3q – TcAChE + Thioflavin T
2ha0 – mAChE + ketoamyltrimethylammonium
2h9y – mAChE + TMTFA
3zlt – mAChE + RVX
3zlu – mAChE + cyclosarin
3zlv – mAChE + tabun + HI-6
4bc0, 4bc1 – mAChE + CBDP
1gpk, 1gpn, 1vot – TcAChE + huperzine
4ey5 – hAChE + huperzine
1gqr – TcAChE + rivastigmine
1gqs – TcAChE + NAP
1e66 – TcAChE + huprine
4a16 – mAChE + huprine
1dx4, 1qon – DmAChE + tacrine derivative
1oce – TcAChE + MF268
1ax9, 1ack – TcAChE + edrophonium
1amn – TcAChE + TMTFA
1acj – TcAChE + tacrine
1u65 – TcAChE + CPT-11
2bag - TcAChE + ganstigmine
2xi4 - TcAChE + aflatoxin
4ara, 4arb, 4a23, 4b7z, 4b80, 4b81, 4b82, 4b83, 4b84, 4b85, 4btl - mAChE + inhibitor
2xuf, 2xug, 2xuh, 2xui, 2xuj, 2xuk, 2xuo, 2xup, 2xuq - mAChE (mutant) + inhibitor
4m0e, 4m0f - hAChE + inhibitor
AChE peripheral site inhibitors conjugating at the surface of the protein
1ku6, 1mah - mAChE + fasciculin 2
1j07 - mAChE + decidium
1n5m - mAChE + gallamine
1n5r - mAChE + propidium
1b41, 1f8u, 4ey8 - hAChE + fasciculin 2
1fss - TcAChE + fasciculin 2
2x8b - hAChE + fasciculin 2 + tabun
4bdt - hAChE + fasciculin 2 + huprine W
AChE bis inhibitors spanning the active site gorge
3i6m – TcAChE + N-piperidinopropyl galanthamine
3i6z - TcAChE + saccharinohexyl galanthamine
1zgb, 1zgc – TcAChE + tacrine (10) hupyridone
2w6c – TcAChE + bis-(-)-nor-meptazinol
2ckm, 2cmf – TcAChE + bis-tacrine
2cek – TcAChE + N-[8-(1,2,3,4-tetrahydroacridin-9-ylthio)octyl]-1,2,3,4-tetrahydroacridin-9-amine
1ut6 - TcAChE + N-9-(1,2,3,4-tetrahydroacridinyl)-1,8-diaminooctane
1odc - TcAChE + N-4-quinolyl-N-9-(1,2,3,4-tetrahydroacridinyl)-1,8-diaminooctane
1w4l, 1w6r, 1w76, 1dx6, 1qti - TcAChE + galanthamine and derivative
4ey6 - hAChE + galanthamine
4ey7 - hAChE + donepezil
1q83, 1q84 - mAChE + TZ2PA6
1h22, 1h23 – TcAChE + bis-hupyridone
1hbj – TcAChE + quinoline derivativev
1e3q – TcAChE + bw284c51
1eve – TcAChE + e2020
1acl – TcAChE + decamethonium
2xud – TcAChE (mutant) + decamethonium
3zv7 - TcAChE + bisnorcymserine
AChE organophosphate inhibitors causing irreversible inhibition
2wu3 – mAChE + fenamiphos and HI-6
2wu4 – mAChE + fenamiphos and ortho-7
2jgf - mAChE + fenamiphos
2wfz, 2wg0, 2wg2, 1som - TcAChE + soman
2wg1 - TcAChE + soman + 2-PAM
2whp, 2whq, 2whr – mAChE + sarin and HI-6
2jgg, 2y2v - mAChE + sarin
2jgl - mAChE + VX and sarin
1cfj - TcAChE + sarin, GB
3dl4, 3dl7 – mAChE + tabun
2jey – mAChE + HLO-7
2c0p, 2c0q - mAChE + tabun
2jez - mAChE + tabun + HLO-7
2jf0 - mAChE + tabun + Ortho-7
2jgh, 2y2u - mAChE + VX
1vxo, 1vxr - TcAChE + VX
2jgi, 2jgm - mAChE + DFP
1dfp - TcAChE + DFP
2jgj, 2jgk, 2jge - mAChE + methamidophos
2gyu - mAChE + HI-6
2gyv - mAChE + Ortho-7
2gyw - mAChE + obidoxime
3gel - TcAChE + methyl paraoxon
2dfp – TcAChE aged
AChE substrate analogues mimicking the binding of the substrate acetylcholine
2ha4 – mAChE (mutant) + acetylcholine
2vja, 2vjb, 2vjc, 2vjd, 2cf5 – TcAChE + 4-oxo-N,N,N-trimethylpentanaminium
2v96, 2v97, 2v98, 2v99 – TcAChE + 1-(2-nitrophenyl)-2,2,2-trifluoroethyl-arsenocholine
2ha2 – mAChE + succinylcholine
2ha3 - mAChE + choline
2ha5 – mAChE (mutant) + acetylthiocholine
2ha6 – mAChE (mutant) + succinylthiocholine
2ha7 – mAChE (mutant) + butyrylthiocholine
2ch4, 2c58 – TcAChE + acetylthiocholine
2c5g – TcAChE + thiocholine
2c5f – TcAChE + substrate analog
2va9 - TcAChE + ‘caged’ arsenocholine
Others...
2j4f – TcAChE + Hg
1vzj – TcAChE tetramerization domain
1jjb – TcAChE + PEG
1qie, 1qif, 1qig, 1qih, 1qii, 1qij, 1qik – TcAChE synchrotron radiation damage
3m3d – TcAChE + Xe
Additional Resources
For additional information, see:
Alzheimer's Disease
AChE inhibitors and substrates
AChE inhibitors and substrates (Part II)
AChE inhibitors and substrates (Part III)
AChE bivalent inhibitors
AChE bivalent inhibitors (Part II)
External Links
- Acetylcholinesterase Tutorial by Karl Oberholser, Messiah College
- PDB Molecule of the Month - Acetylcholinesterase
- Movies: X-ray Damage in ACh & Nature's Vacuum Cleaner by R. Gillilan, Cornell Univ
References
- ↑ Sussman JL, Harel M, Frolow F, Oefner C, Goldman A, Toker L, Silman I. Atomic structure of acetylcholinesterase from Torpedo californica: a prototypic acetylcholine-binding protein. Science. 1991 Aug 23;253(5022):872-9. PMID:1678899
- ↑ Botti SA, Felder CE, Lifson S, Sussman JL, Silman I. A modular treatment of molecular traffic through the active site of cholinesterase. Biophys J. 1999 Nov;77(5):2430-50. PMID:10545346
- ↑ 3.0 3.1 Raves ML, Harel M, Pang YP, Silman I, Kozikowski AP, Sussman JL. Structure of acetylcholinesterase complexed with the nootropic alkaloid, (-)-huperzine A. Nat Struct Biol. 1997 Jan;4(1):57-63. PMID:8989325
- ↑ Greenblatt HM, Kryger G, Lewis T, Silman I, Sussman JL. Structure of acetylcholinesterase complexed with (-)-galanthamine at 2.3 A resolution. FEBS Lett. 1999 Dec 17;463(3):321-6. PMID:10606746
- ↑ Harel M, Schalk I, Ehret-Sabatier L, Bouet F, Goeldner M, Hirth C, Axelsen PH, Silman I, Sussman JL. Quaternary ligand binding to aromatic residues in the active-site gorge of acetylcholinesterase. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):9031-5. PMID:8415649
- ↑ Bar-On P, Millard CB, Harel M, Dvir H, Enz A, Sussman JL, Silman I. Kinetic and structural studies on the interaction of cholinesterases with the anti-Alzheimer drug rivastigmine. Biochemistry. 2002 Mar 19;41(11):3555-64. PMID:11888271
- ↑ Haviv H, Wong DM, Greenblatt HM, Carlier PR, Pang YP, Silman I, Sussman JL. Crystal packing mediates enantioselective ligand recognition at the peripheral site of acetylcholinesterase. J Am Chem Soc. 2005 Aug 10;127(31):11029-36. PMID:16076210 doi:http://dx.doi.org/10.1021/ja051765f
- ↑ 8.0 8.1 Ravelli RB, Raves ML, Ren Z, Bourgeois D, Roth M, Kroon J, Silman I, Sussman JL. Static Laue diffraction studies on acetylcholinesterase. Acta Crystallogr D Biol Crystallogr. 1998 Nov 1;54(Pt 6 Pt 2):1359-66. PMID:10089512
- ↑ 9.0 9.1 Harel M, Sonoda LK, Silman I, Sussman JL, Rosenberry TL. Crystal structure of thioflavin T bound to the peripheral site of Torpedo californica acetylcholinesterase reveals how thioflavin T acts as a sensitive fluorescent reporter of ligand binding to the acylation site. J Am Chem Soc. 2008 Jun 25;130(25):7856-61. Epub 2008 May 31. PMID:18512913 doi:http://dx.doi.org/10.1021/ja7109822
- ↑ Greenblatt HM, Guillou C, Guenard D, Argaman A, Botti S, Badet B, Thal C, Silman I, Sussman JL. The complex of a bivalent derivative of galanthamine with torpedo acetylcholinesterase displays drastic deformation of the active-site gorge: implications for structure-based drug design. J Am Chem Soc. 2004 Dec 1;126(47):15405-11. PMID:15563167 doi:http://dx.doi.org/10.1021/ja0466154
- ↑ Kryger G, Silman I, Sussman JL. Structure of acetylcholinesterase complexed with E2020 (Aricept): implications for the design of new anti-Alzheimer drugs. Structure. 1999 Mar 15;7(3):297-307. PMID:10368299
- ↑ Sanson B, Nachon F, Colletier JP, Froment MT, Toker L, Greenblatt HM, Sussman JL, Ashani Y, Masson P, Silman I, Weik M. Crystallographic Snapshots of Nonaged and Aged Conjugates of Soman with Acetylcholinesterase, and of a Ternary Complex of the Aged Conjugate with Pralidoxime (dagger). J Med Chem. 2009 Jul 30. PMID:19642642 doi:10.1021/jm900433t
- ↑ Paz A, Roth E, Ashani Y, Xu Y, Shnyrov VL, Sussman JL, Silman I, Weiner L. Structural and functional characterization of the interaction of the photosensitizing probe methylene blue with Torpedo californica acetylcholinesterase. Protein Sci. 2012 Jun 1. doi: 10.1002/pro.2101. PMID:22674800 doi:10.1002/pro.2101
Treatments:AChE Inhibitor References
Treatments:Alzheimer's Disease
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Joel L. Sussman, Alexander Berchansky, David Canner, Eran Hodis, Clifford Felder, Jaime Prilusky, Harry Greenblatt, Yechun Xu