Molecular Playground/DnaK
From Proteopedia
| Line 5: | Line 5: | ||
Molecular Playground banner: DnaK, a central hub in maintaining proteostasis in E. coli | Molecular Playground banner: DnaK, a central hub in maintaining proteostasis in E. coli | ||
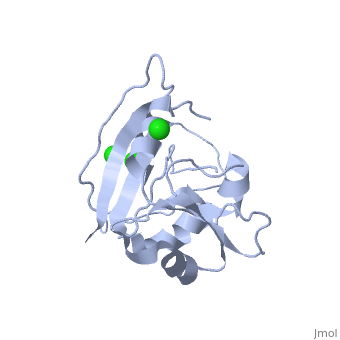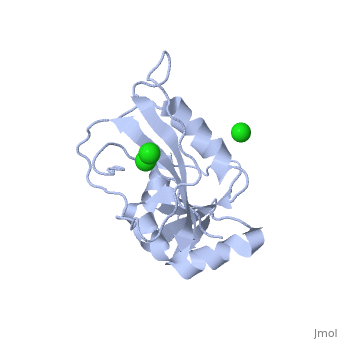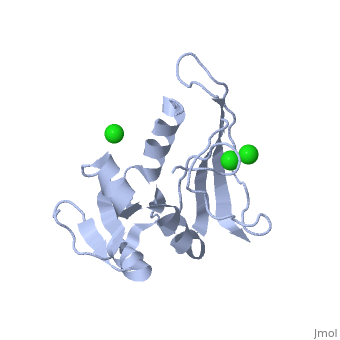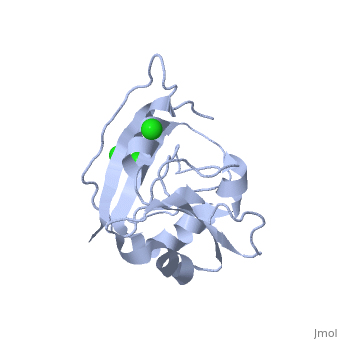
| - | <scene name=' | + | <scene name='60/609794/Adp-dnak_1/1'>DnaK in the extended ADP_bound conformation</scene> |
The E. coli Hsp70, DnaK, is a crucial protein chaperone whose function is to reduce bind exposed hydrohpobic residues of unfolded proteins, which prevents aggregation and rescues the nascent chain from kinetic traps along the folding pathway. Hsp70 protein chaperones switch between an ATP-bound, low-substrate affinity form and an ADP-bound, high substrate affinity form during their allosteric cycle. (EC: 1.5.1.3). Hsp70 protein chaperones are ubiquitously found in almost all known organisms and cell types and represent a potential target for anti-cancer and neurodegenerative therapies. <scene name='User:Karan_Hingorani/sandbox_2/Apo_dhfr/5'>Apo-DHFR</scene> free of any of its ligands is displayed here.[http://www.ncbi.nlm.nih.gov/pubmed/2185835?dopt=Abstract] | The E. coli Hsp70, DnaK, is a crucial protein chaperone whose function is to reduce bind exposed hydrohpobic residues of unfolded proteins, which prevents aggregation and rescues the nascent chain from kinetic traps along the folding pathway. Hsp70 protein chaperones switch between an ATP-bound, low-substrate affinity form and an ADP-bound, high substrate affinity form during their allosteric cycle. (EC: 1.5.1.3). Hsp70 protein chaperones are ubiquitously found in almost all known organisms and cell types and represent a potential target for anti-cancer and neurodegenerative therapies. <scene name='User:Karan_Hingorani/sandbox_2/Apo_dhfr/5'>Apo-DHFR</scene> free of any of its ligands is displayed here.[http://www.ncbi.nlm.nih.gov/pubmed/2185835?dopt=Abstract] | ||
Revision as of 17:44, 19 November 2014
|
One of the CBI Molecules being studied in the University of Massachusetts Amherst Chemistry-Biology Interface Program at UMass Amherst and on display at the Molecular Playground.
Molecular Playground banner: DnaK, a central hub in maintaining proteostasis in E. coli
The E. coli Hsp70, DnaK, is a crucial protein chaperone whose function is to reduce bind exposed hydrohpobic residues of unfolded proteins, which prevents aggregation and rescues the nascent chain from kinetic traps along the folding pathway. Hsp70 protein chaperones switch between an ATP-bound, low-substrate affinity form and an ADP-bound, high substrate affinity form during their allosteric cycle. (EC: 1.5.1.3). Hsp70 protein chaperones are ubiquitously found in almost all known organisms and cell types and represent a potential target for anti-cancer and neurodegenerative therapies. free of any of its ligands is displayed here.[1]
Contents |
Structure
DnaK is a relatively large 638 residue protein of approximately 70 kDa. It has an a/b structure with eight central B strands and four helices. The protein can be thought to be made up of two subdomains, divided by the active site cleft. The which consists of residues 38-88 and the major subdomain comprised of about 100 residues. Three loops can be found in the major subdomain and they make up about 50% of this domain. They are the (residues 9-24), the (residues 116-132)and the (residues 142-150). The Met20 loop assumes different conformations during catalysis and accomodation of ligands is made possible by the 'hinge bending' motion about Lys 38 and Val 88 of the Adenosine binding domain.[2]
Catalysis
DHFR catalyzes the reduction of 7,8-dihydrofolate to 5,6,7,8-tetrahydrofolate using reduced Nicotinamide Adenine Dinucleotide Phosphate (NADPH). This system has been key model to decipher enzyme catalysis and the intermediates of the catalytic cycle have been identified by crystallography. CPMG relaxation NMR experiments have also revealed that intermediates in the catalytic cycle exist in equilibrium with the preceding or following intermediate. Thus the binding of ligands seems to happen via a conformational selection rather than the traditional view of induced fit which is used to explain conformation change on ligand binding.[3]. shows the ligands Dihydrofolate and NADP+ positioned in the active site cleft.[4]
Drug Target
Since DHFR is so critically positioned in the metabolic homeostasis of all organsims it has been the target of choice for anti microbial and anti cancer therapy. Inhibitors of this enzyme are essentially folate mimics, methotrexate which was first designed to inhibit and used as therapy for cancer and autoimmune disorders. Another folate mimic Trimethoprim was developed as an anti bacterial agent, having much more binding specificity to bacterial DHFR than its mammalian counterpart. Both drugs bind in the active site of the enzyme and are irreversibly bound thus ablating enzyme activity.[5] [6].
3D structures of DHFR
See Also
The wikipedia link on DHFR is also pretty useful for a general background.[[7]]
References
1. Bystroff C. et al. Biochemistry 1990
2. Schnell JR. et al. Annu Rev Biophys Biomol Struct. 2004
3. Boehr DD. et al. Science 2006
4. Bystroff C. et al. Biochemistry 1990
5. Dauber-Osguthorpe P et al. Proteins 1988
6. Cody V. et al. Acta Crystallogr D Biol Crystallogr. 2005